- Submissions

Full Text
COJ Nursing & Healthcare
L-Arginine and Nitric Oxide Level among Pregnant Women in Sokoto, Nigeria
Osaro Erhabor1*, Hashimu Bello Bunza1, Isaac Zama1, Yakubu Ahmad2, Tosan Erhabor3 and Knox Van Dyke4
1 Department of Hematology,UsmanuDanfodiyo University, Nigeria
2 Department of Obstetrics and Gynaecology,UsmanuDanfodiyo University, Nigeria
3 Medical Laboratory Science Council of Nigeria, Nigeria
4 Department of Biochemistry and Molecular Pharmacology, West Virginia School of Medicine, USA
*Corresponding author: Osaro Erhabor, Department of Haematology UsmanuDanfodiyo University Sokoto, Nigeria, Tel: +234-813-962-5990; Email:n_osaro@yahoo.com
Submission: April 13, 2018;Published: July19, 2018

ISSN: 2577-2007Volume3 Issue5
Abstract
Pregnancy is a state of relative arginine deficiency imposed by the increased formation of nitric oxide to support the adaptive vasodilatation of pregnancy. This case control study involved a total of 74 pregnant (subjects) and 50 non-pregnant women (controls). The subjects were aged 19 years to 40 years with mean age of 27.6±5.0 years. The mean values of L-arginine level among 74 pregnant subjects was (173±63.7μmol/L) compared to (137.9±89.5μmol/L) among 50 non-pregnant control group is (p=0.271). The mean values of Nitric Oxide level among the pregnant subjects (334±80.6μmol/L) was significantly higher that that among the non-pregnant controls (280.5±130μmol/L) (p=0.002). The mean values of L-arginine and nitric oxide were compared among the subjects based on trimester. There was no significance difference in the L-arginine and nitric oxide levels (175±63.9) and (303.5±118) among pregnant women within their first trimester and second trimester (185.5±61) and (344±74) (p=0.337 and 0.750) respectively. There was no significance difference in the mean values of L-arginine and nitric Oxide among pregnant women in the second (185.5±61) and (344±74) and third trimester of pregnancy (161±65.5) and (328±83) (p=0.115 and 0.409) respectively.
Similarly, there was no significance difference in the mean values of L- arginine and nitric Oxide among pregnant women in the first (175±63.9) and (303.5±118) and third semester (161±65.5) and (328±83) (p=0.704 and 0.407). The mean value of L-arginine and nitric oxide was compared based on ethnicity of the subjects. L-arginine and nitric oxide levels among Hausa/Fulani ethnic group is (167.8±63) and (330.7±75) respectively. The mean value of L-arginine and nitric oxide level among Yoruba ethnic group is (168.5.5±76) and (411±57) respectively. While the mean values of L-arginine and nitric oxide among Igbo ethnic group is (217±57) and (237.8±128) respectively. There were no significant differences in the L-arginine levels among subjects based on ethnicity (p>0.05). The nitric oxide level was significantly higher among Hausa/Fulani (330.7±75) compared to the Igbo ethnic group (237.8±124) (p=0.046). The mean values of L-arginine and nitric oxide was compared based on parity. The mean values of L-arginine and nitric oxide among pregnant women who are carrying their first pregnancy is (148±55.7) and (348.8±75.5) respectively. The mean values of L-arginine and nitric oxide among para 1,2,3,4, and >4 pregnant women were; (190.9±72.9) and (318.7±56), (151±56.8) and (341.6±81.7), (201±48) and (306±91.8), (146±67) and (346±57.8) and (181±70) and (348±98.8) respectively.
There was a statistically significant difference in the mean L-arginine levels between para 2 (151±56.8) and 3 women (201±48) (p=0.029). Similarly, the mean L-arginine levels was significantly higher among para 3 (201±48) compared to para 0 pregnant women (148±55.7) (p=0.010). There were no statistically significant differences in the mean L-arginine and nitric oxide based on the on the occupation of husband and the level of educational attainment of the subjects (p>0.05). In conclusion, finding from this study confirmed that the level of L-arginine and nitric oxide is higher among pregnant subjects compared to non-pregnant controls. Gestational age (trimester), ethnicity, parity, and socio-economic status was also found to have effect on the level of L-arginine and nitric oxide. We recommend that further research need to be conducted involving a larger population of pregnant women with different gestational age, parity, ethnicity and socio-economic status.We also recommend that pregnant women found to have low level of L-arginine and nitric oxide should be given L-arginine supplements as a prophylaxis. We recommend that public enlightenment programme should be implemented to enlighten pregnant women on how to maintain the normal level of L-arginine and nitric oxide through taking a balance diet and food that serve as a source of L-arginine. We also recommend that pregnant women should be offered a routine L-arginine and nitric oxide test to monitor their level of L-arginine and nitric oxide.
Keywords: L-Arginine; Nitric oxide; Pregnant women; Sokoto; Nigeria
Introduction
Pregnancy, gravidity or gestation, is the time during which one or more offspring develops inside a woman. Maternal mortality continues to be the major cause of death among women of reproductive age in many countries and remains a serious public health issue especially in developing countries [1]. According to Shah and Say [2] maternal death is defined as the death of a woman while pregnant or within 42 days of termination of pregnancy, irrespective of the duration and site of the pregnancy, from any cause related to or aggravated by the pregnancy or its management but not from accidental or incidental causes. Globally, the estimated number of maternal deaths worldwide in 2005 was 536,000 as against 529,000 in 2000. According to WHO factsheet (2008) [3], 1500 women die from pregnancy- related complications every day. Most of these deaths occur in developing countries, and most are avoidable. While 25 percent of females of reproductive age lived in developed countries, they contributed 1 percent of maternal deaths worldwide [4]. A total of 99 percent of all maternal deaths occur in developing countries. More than half of these deaths occur in sub- Saharan Africa and one third in South Asia. The maternal mortality ratio in developing countries is 450 maternal deaths per 100,000 live birth versus 9 per 100,000 live births in developed countries. Fifteen countries with maternal mortality ratios of at least 1000 per 100,000 live births are all sub Saharan except Afghanistan and India [5].
Pregnancy-related pre-eclampsia and eclampsia are among the leading causes of maternal and neonatal morbidity and mortality [6]. Despite growing knowledge of the pathophysiology of pregnancy induced hypertensive disorders, no preventive measures have been shown to be effective [7]. The underlying cause of pre-eclampsia/ eclampsia is thought to be abnormal placentation, characterized by defective invasion of trophoblast cells and remodeling of the uterine vasculature [8] resulting in reduced utero-placental perfusion, which leads to activation of mechanisms promoting maternal vasoconstriction and activation or damage of endothelial cells [9]. The endothelium is believed to be a primary target of mediators generated by the placenta. Damage is amplified by other factors such as reactive oxygen species [10]. During pregnancy, the woman undergoes many physiological changes, which are entirely normal, including cardiovascular, haematologic, metabolic, renal and respiratory changes that become very important in the event of complications [11].
Arginine is an α amino acid [12]. It was first isolated in 1886. In mammals, arginine is classified as a semi- essential or conditionally essential amino acid, depending on the developmental stage and health status of the individual [13]. Preterm infants are unable to synthesize or create arginine internally, making the amino acid nutritionally essential for them [14]. Most healthy people do not need to supplement with arginine because their body produces sufficient amounts [14]. Individuals with poor nutrition or certain physical conditions may be advised to increase their intake of foods containing arginine. Arginine is found in a wide variety of foods. It is a precursor for the synthesis of nitric oxide (NO) [15]. Non-Larginine derived no can be generated by the nitrate-nitrite-nitric oxide pathway that is monitored through saliva testing. It helps decrease blood pressure in clinical hypertensive subjects [16-18]. NO-mediated decrease in blood pressure is influenced by both the L-arginine-dependent nitric oxide synthase pathway and non-Larginine or alternative pathway through nitrate-rich foods such as beets and spinach.
Nitric oxide (nitrogen oxide) or nitrogen monoxide [19] is a molecular, chemical compound with chemical formula of NO that is a colourless gas under standard conditions. Nitric oxide is a free radical (its bonding structure includes an unpaired electron) [20] and it is in the class of heteronuclear diatomic molecules. Previous report indicated that infusion of L-arginine into pregnant women with complicated intrauterine growth retardation resulted in reduced myometrial activity [21,22]. These observations raised the possibility that supplemental L-arginine in the diet could provide a source of substrate for nitric oxide synthesis during pregnancy, which could promote vasodilatation. More studies are needed to provide more evidence on the use of L-arginine and other nitric oxide donators for preventing pre-eclampsia [23]. On the other hand, evidence of endothelial damage mediated by reactive oxygen species has been proposed as the mechanism of endothelial damage in pre-eclampsia [24]. Consequently, antioxidants have been proposed as prophylactic agents for pre-eclampsia and several trials with antioxidants including vitamin C and tocopherols in preeclampsia have been published [25].
Nitric oxide is a potent endothelium-derived vasodilator [26] and defective synthesis of nitric oxide has been documented in pre-eclampsia [27]. The main site of production of nitric oxide (by nitric oxide synthase) is endothelial cells, which uses circulating L-arginine as a substrate. Hence, the local availability of this amino acid may be critical to the endothelial adaptive regulatory mechanisms opposing the vasoconstrictors in pre-eclampsia.Larginine is considered to be a semi-essential amino acid because under increased demands endogenous synthesis is not sufficient to meet requirements [28]. Moreover, pregnancy has been reported to be a state of relative arginine deficiency [29] imposed by the increased formation of nitric oxide, supporting the adaptive vasodilatation of pregnancy and use of L-arginine by the foetus [30]. Pre-eclampsia is also associated with increased concentration of factors that inhibit nitric oxide production. The aim of the study is to determine the level of L-arginine and nitric oxide among pregnant women attending antenatal clinic (ANC) in UsmanuDanfodiyo University Teaching Hospital Sokoto, Nigeria.
Material and Methods
Study siteandparticipating hospital
The sample for this research was collected from consecutivelyrecruited pregnant women visiting the antenatal clinic (ANC) of UsmanuDanfodiyo University Teaching Hospital (UDUTH) and the testing was carried out at the Haematology Laboratory in the Faculty of Medical Laboratory Sciences, UsmanuDanfodiyo University Sokoto in collaboration with the Haematology Laboratory of UsmanuDanfodiyo University Teaching Hospital. Sokoto State is located in the extreme Northwest of Nigeria between longitude 050 111 and 130 031 East and latitude 130 001 and 130 061 North. The state shares border with the Republic of Niger to the North, Kebbi state to the West and Southeast and Zamfara to the East. The state covers a land area of about 60.33km2. Sokoto is, on the whole, a very hot area. However, the maximum daytime temperature for most of the year is generally under 40ᵒC(104.00F). The warmest months are February to April when day time temperatures can exceed 45ᵒC (113.00F). The rainy season is from June to October during which showers are a daily occurrence. There are two major seasons, wet and dry which are distinct. The indigenous inhabitant of the area is the Hausa and Fulani. Other ethnic group resident in the area include Igbo, Ebira, Yoruba, Igala etc. Hausa is the commonly spoken language. Traders form the greater percentage of the population, while the rest are civil servants, farmers, artisans and others.
Study subjects
The study involved 74 consecutively-recruited pregnant women aged ≥18 years visiting the antenatal clinic in UsmanuDanfodiyo University Teaching Hospital, Sokoto, Nigeria. Fifty (50) agedmatched non- pregnant women has been monitored as controls.
Study design
This study has been designed as a case control study involving 74 pregnant study subjects and 50 non-pregnant and age-matched women who served as controls.
Eligibility criteria
All adult female (≥18 years), consenting, and confirmed pregnant women were eligible to participate as subjects in this study.
Exclusion criteria
All non-adult female (< 18 years), non-pregnant or nonconsenting pregnant women were excluded from participation as subjects in this study.
Informed consent
Verbal informed and written consent have been sought from study participants. Ethical clearance was sought from the ethical committee of UsmanuDanfodiyo University Teaching Hospital (UDUTH) Sokoto.
Questionnaire
A questionnaire was distributed among all the participants to obtain socio-demographic and obstetric-related data.
Blood sample collection and preparation
Five milliliters of venous blood sample were collected from each study subject and control participant by venipuncture into EDTA anticoagulated tubes. Blood samples are then centrifuged to obtain plasma. Plasma sample has been used for L-arginine and nitric oxide testing. L-arginine has been determined using L-arginine Enzyme Linked Immunosorbent Assay (ELISA) kit (Immunediagnostik, Germany). Nitric oxide has been measured using a colorimetric method manufactured by ENZO Life Sciences (United Kingdom).
Statistical analysis
Data was analyzed using relevant statistical software (SPSS Version 20). Data collected were descriptive. Results obtained were expressed as mean and standard deviation. Statistical analysis includes student t-test and analysis of variance. Differences in values based on socio-demographic and obstetric variables of subjects was determined and compared statistically. A p-value of ≤0.05 was considered significant in all statistical comparison.
Result
Table 1:L-arginine and nitric oxide levels among pregnant (subjects) and non-pregnant women (controls).
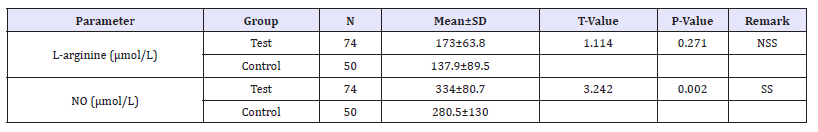
N=Number; SD=Standard Deviation; DF =Degree Of Freedom; SS=Statistical Significance; NO=Nitric Oxide
This case control study involved a total of 74 pregnant (subjects) and 50 non-pregnant women (controls). The subjects were aged 19 years to 40 years with mean age of 27.6±5.0 years. The mean values of L-arginine level among 74 pregnant subjects was (173±63.7) and the mean values of L-arginine level among 50 non-pregnant control group is (137.9±89.5) (p=0.271). The mean values of Nitric Oxide level among the pregnant subjects (334±80.6) was significantly higher that that among the non-pregnant controls is (280.5±130) (p=0.002). Table 1 shows the L-arginine and nitric oxide levels among pregnant (subjects) and non-pregnant women (controls).
Table 2:Effect of gestational age on the level of L-arginine and nitric oxide among pregnant women.
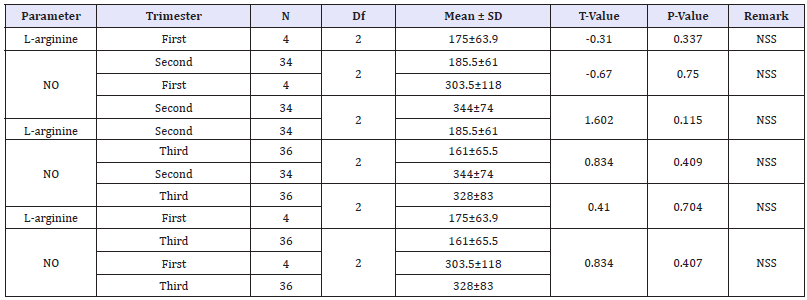
N=Number; SD=Standard Deviation; DF=Degree Of Freedom; SS=Statistical Significance; NO=Nitric Oxide
The mean values of L-arginine and nitric oxide were compared among the subjects based on trimester. There was no significance difference in the L-arginine and nitric oxide levels (among pregnant women within their first trimester 175±63.9) and (303.5±118) and second trimester (185.5±61) and (344±74) (p=0.337 and 0.750) respectively. There was no significance difference in the mean values of L- arginine and nitric Oxide among pregnant women in the second (185.5±61) and (344±74) and third trimester of pregnancy (161±65.5) and (328±83) (p=-0.115 and 0.409) respectively. Similarly, here was no significance difference in the mean values of L- arginine and nitric Oxide among pregnant women in the first (175±63.9) and (303.5±118) and third semester (161±65.5) and (328±83) (p=0.704 and 0.407). Table 2 show the mean values of L-arginine and nitric oxide was compared based on trimester.
The mean value of L-arginine and nitric oxide was compared based on ethnicity of the subjects. L-arginine and nitric oxide levels among Hausa/Fulani ethnic group is (167.8±63) and (330.7±75) respectively. The mean value of L-arginine and nitric oxide level among Yoruba ethnic group is (168.5.5±76) and (411±57) respectively. While the mean values of L-arginine and nitric oxide among Igbo ethnic group is (217±57) and (237.8±128) respectively. There were no significant differences in the L-arginine levels among subjects based on ethnicity (p>0.05). The nitric oxide level was significantly higher among Hausa/Fulani (330.7±75) compared to the Igbo ethnic group (237.8±124) (p=0.046). Table 3 shows the effect of ethnicity on the level of L-arginine and nitric oxide among pregnant women.
Table 3:Effect of ethnicity on the level of L-arginine and nitric oxide among pregnant women.
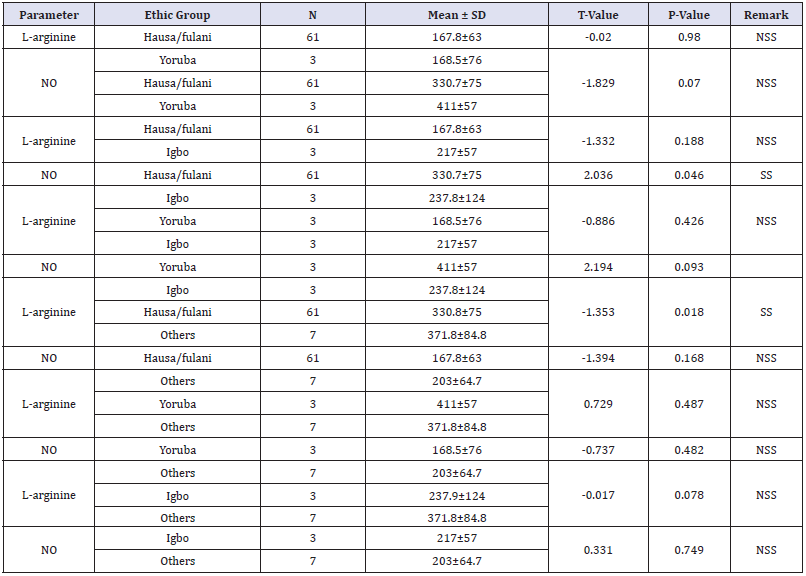
The mean values of L-arginine and nitric oxide was compared based on parity. The mean values of L-arginine and nitric oxide among pregnant women who are carrying their first pregnancy is (148±55.7) and (348.8±75.5) respectively. The mean values of L-arginine and nitric oxide among para 1,2,3,4, and >4 pregnant women were; (190.9±72.9) and (318.7±56), (151±56.8) and (341.6±81.7), (201±48) and (306±91.8), (146±67) and (346±57.8) and (181±70) and (348±98.8) respectively. There was a statistically significant difference in the mean L-arginine levels between para 2 (151±56.8) and 3 women (201±48) (p=0.029). Similarly, the mean L-arginine levels was significantly higher among para 3 (201±48) compared to para 0 pregnant women (148±55.7) (p=0.010). Table 4 show the mean values of L-arginine and nitric oxide was compared based on parity. There were no statistically significant differences in the mean L-arginine and nitric oxide based on the on the occupation of husband and the level of educational attainment of the subjects (p>0.05) as shown in Table 5 & 6 shows the mean L-arginine and nitric oxide based on the on the occupation of husband and the level of educational attainment of the subjects.
Table 4:Effect of parity on the level of L-arginine and nitric oxide among pregnant women.
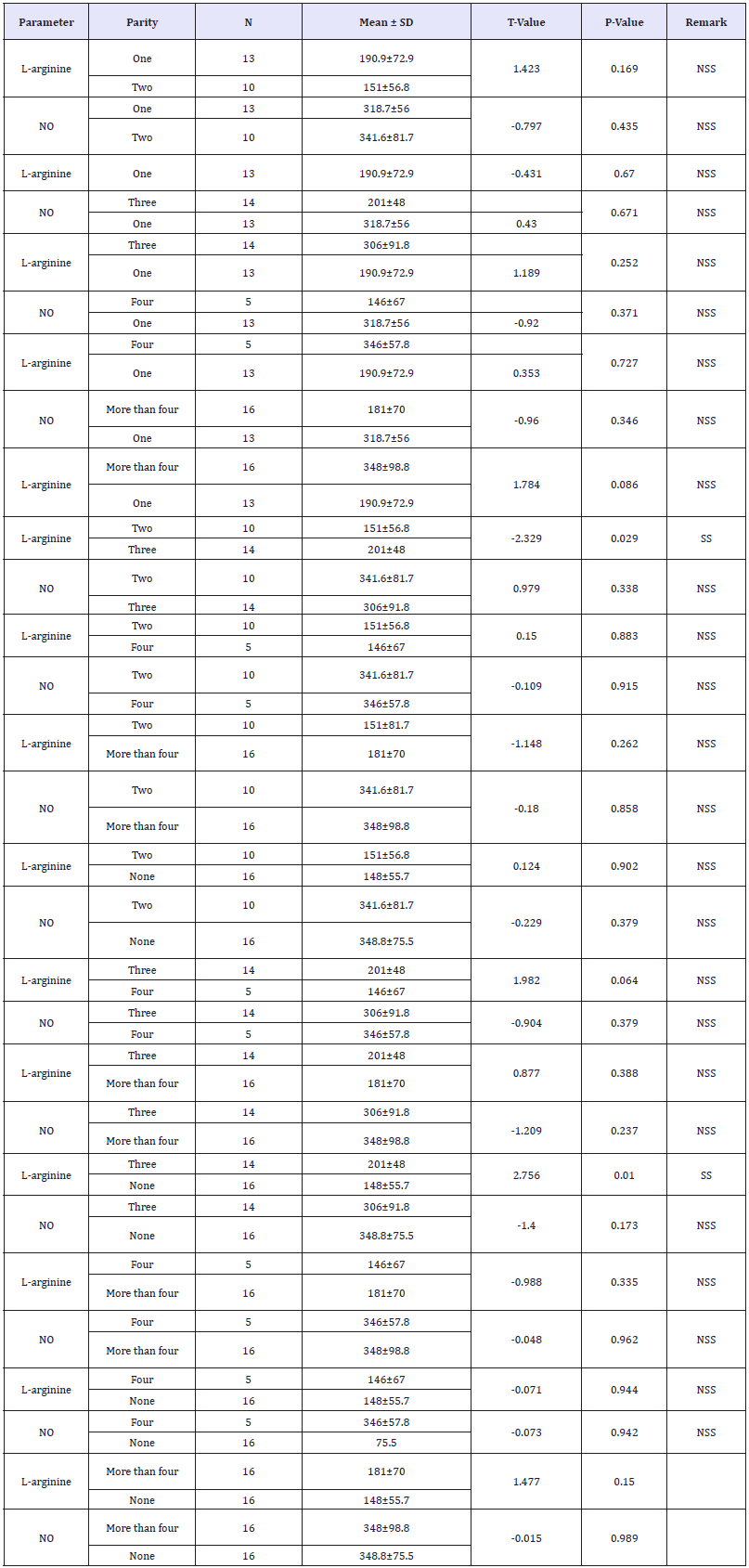
Table 5:Occupation of husband on L-arginine and nitric oxide level among pregnant women.
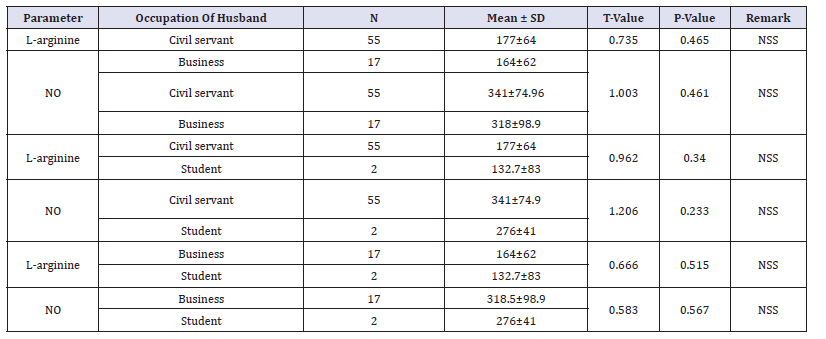
Table 6:Level of education on L-arginine and nitric oxide among pregnant women.

Discussion
In this study, we found that the L-arginine level was marginally higher among pregnant women compared to non-pregnant women. The difference however was not statistically significant (p=0.271). Nitric oxide (NO) level was found to be significantly higher among pregnant women compared to non-pregnant controls (p=0.002). This finding is in agreement with previous report [31,32], which indicated that serum NO production was increased in normal pregnancy. Arginine, a nutritionally essential amino acid for the fetus[33] is a precursor for synthesis of NO [34]. Consequently, it may play a critical role in foetal nutrition and oxygenation resulting in improvement of IUGR pregnancy, enhancing birth weight and decreasing neonatal morbidity and mortality [35]. L-arginine may have a role in the prevention and treatment of pregnancyinduced hypertension [36,37]. Oral L-arginine supplementation significantly lowers both systolic and diastolic BP [38]. L-arginine as an antihypertensive agent for gestational hypertension especially in view of the other beneficial effects nitric oxide donors display in pregnancy [39]. Finding from this study seems a justification to routinely prescribe L-arginine particularly to reduce the incidence of pregnancy induced hypertensive disorders including pre-eclampsia and eclampsia. Previous report [40] indicated that supplementation during pregnancy with a medical food containing Larginine and antioxidant vitamins reduced the incidence of preeclampsia in a population at high risk of the condition.
Pregnancy has been reported to be a state of relative arginine deficiency [41] imposed by the increased formation of nitric oxide, supporting the adaptive vasodilatation of pregnancy, and use of L-arginine by the foetus [42]. Previous report in animal model indicates that L-arginine could have a beneficial effect on haemodynamics[43,44]. Administration of L-arginine has been shown to improve vascular function in people with atherosclerosis and peripheral vascular disease [45,46]. Facchinetti et al. [47] and Neri et al. [48] infused L-arginine into women whose pregnancies were complicated by intrauterine growth retardation and reported reduced myometrial activity. These observations raised the possibility that supplemental L-arginine in the diet could provide a source of substrate for nitric oxide synthesis during pregnancy, which could promote vasodilatation. Nitric oxide is a potent endothelium derived vasodilator. Normal pregnancy leads to profound maternal hemodynamic changes, including increased blood volume and vasodilatation. Several vasodilator mediators are implicated, including nitric oxide (NO) [49].Nitric oxide production is enhanced in severe preeclampsia, possibly as a compensatory phenomenon for the increased synthesis and release of vasoconstrictors and platelet-aggregating agents [50].
Pregnancy-induced hypertension (PIH) is a pregnancy-specific hypertensive disorder, which can complicate 7-10% of pregnancies [51]. One of the PIH causes is excessive production of free radicals that induce arterial vasoconstriction.The other factors predisposing pregnant women to PIH include multiple pregnancy, foetal male gender, young maternal age (under 18 years of age in primiparas), advanced maternal age (over 40 years of age in multiparas), obesity, genetic load, chronic stress and excess sodium in diet [52-54].
The effect of gestational age on the level of L-arginine and nitric oxide was also considered in this study. There were no statistically significant differences in the L-arginine and nitric oxide of the subjects based on their gestational age. Our finding is consistent with previous reports [55,56] who observed that there were no changes in no production during normal pregnancy compared to the non-pregnant state. However, our finding is at variance with a previous report [57] which indicated that maternal circulating nitrite level decreased with advancing gestation. Our finding is also at variance with a previous report [58] which indicated that no production increases with advancing gestation during normal pregnancy and decreases in preeclampsia. These observations suggest that the status of no biosynthesis in women during normal pregnancy remains controversial. These discrepancies may derive from methodological shortcomings for measuring plasmanoconcentrations. Dietary intake of nitrate can affect the blood level of NO. Many of the studies relied on the measurement of no in the plasma; however, the plasma level is influenced by the clearance, as well as the production, of nometabolites [59].
Our study also considered the effect of ethnicity on the level of L-arginine and nitric oxide among pregnant women. Nitric oxide level was found to be significantly higher among Hausa/Fulani than among Igbos (p=0.04). The mechanisms underlying these racial disparities may be multifactorial and involve genetic factors, socioeconomic status, nutritional status, psychosocial stressors/ risks, and other environmental factors. Reduced nitric oxide (NO) bioavailability has been recognized as a hallmark of preeclampsia and low NO bioavailability has been reported in non-pregnant Black people [60]. A previous report indicates that microvascular characteristics of Black pregnant women may contribute to the greater risk for pregnancy-related hypertensive disorders in this population subgroup [61].
In this research study, the effect of socio-economic status of occupation of the husbands of the pregnant subjects and the level of educational attainment of the subjects on the L-arginine and nitric oxide of the subjects was determined. There were no statistically significant differences in the L-arginine and nitric oxide based on the occupation of the husbands and the level of educational attainment of the subjects. Healthy and balanced diet is quite important in life time and during pregnancy in particular. Previous report indicate that the levels of literacy affect the level of nutritional attainment of pregnant women [62] Education can play a significant role on nutrition and food consumption and can resolve safety issues particularly among pregnant women [63].
Conclusion
In conclusion, finding from this study confirmed that the level of L-arginine and nitric oxide is higher among pregnant controls compared to pregnant women. Gestational age (trimester), ethnicity, parity, and socio-economic status were also found to have effect on the level of L-arginine and nitric oxide.
Recommendation
We recommend that a further research need to be conducted involving a larger population of pregnant women with different gestational age, parity, ethnicity and socio-economic status.We also recommend that pregnant women found to have low level of L-arginine and nitric oxide should be given L-arginine supplements as a prophylaxis. We recommend that public enlightenment programme should be created to enlighten pregnant women on how to maintain the normal level of L-arginine and nitric oxide through taking a balance diet and food that serve as a source of L-arginine. We also recommend that pregnant women should be offered a routine L-arginine and nitric oxide test to monitor their level of L-arginine and nitric oxide.
Limitation
One of the limitations of this study is that we did not compare the level of NO and L-arginine among the non-pregnant women of different ethnicity and socioeconomic status. We also did not subject the subjects and control non-pregnant woman recruited in this study to fasting for 12 hours prior blood taking because we did not want to put the pregnant subject through undue stress.
References
- World Health Organization (2007) Regional committee for Africa resolution, health financing: a strategy for the African region.
- Shah IH, Say L (2007) Maternal mortality care from 1990-2005: uneven but important gains. Reprod Health Matters 15(30): 17-27.
- World Health Organization (2008) Factsheet, maternal mortality, Department of making pregnancy safer, Switzerland.
- Alvarez JL, Gil R, Hernández V, Gil A (2009) Factors associated with maternal mortality in Sub-Saharan Africa: an ecological study. BMC Public Health 9: 462.
- WHO, UNICEF, UNFPA (2015) World bank group, and the united nations population division. Trends in Maternal Mortality: 1990 to 2015. World Health Organization, Switzerland.
- Ronsmans C, Graham W (2006) Maternal mortality: Who, when, where, and why. Lancet 368(9542): 1189-1200.
- Meher S, Duley L (2007) Nitric oxide for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev 18(2): CD006490.
- Mignini LE, Villar J, Khan KS (2006) Mapping the theories of preeclampsia: The need for systematic reviews of mechanisms of the disease. Am J Obstet Gyneco 194(2): 317-321.
- Sibai B, Dekker G, Kupferminc M (2005) Pre-eclampsia. Lancet 365(9461): 785-799.
- Davidge S (1998) Oxidative stress and altered endothelial cell function in pre-eclampsia. Semin Reprod Endocrinol 16(1): 65-73.
- Reem M, Sana A, Anu G, Rocco C (2012) A comprehensive review of hypertension in pregnancy. Journal of Pregnancy 4: 105918.
- International Union of Pure and Applied Chemistry (IUPAC) (1984). Nomenclature and symbolism for amino acids and peptides”. J Biochem 138(1): 9-37.
- Tapiero H, Mathé G, Couvreur P, Tew KD (2002) L-Arginine. Biomed Pharmacother 56(9): 439-445.
- Wu G, Jaeger LA, Bazer FW, Rhoads JM (2004) Arginine deficiency in preterm infants: biochemical mechanisms and nutritional implications. The Journal of Nutritional Biochemistry 15(8): 442-451.
- Andrew PJ, Mayer B (1999) Enzymatic function of nitric oxide synthases. Cardiovasc Res 43(3): 521-531.
- Kapil V, Haydar SM, Pearl V, Lundberg JO, Weitzberg E, et al. (2012) Physiological role for nitrate-reducing oral bacteria in blood pressure control. Free Radic Biol Med 55: 93-100.
- Rajapakse NW, De Miguel C, Das S, Mattson DL (2008) Exogenous L-arginine ameliorates angiotensin II-induced hypertension and renal damage in rats. Hypertension 52(6): 1084-1090.
- Lidder S, Webb AJ (2013) Vascular effects of dietary nitrate (as found in green leafy vegetables and beetroot) via the nitrate‐nitrite‐nitric oxide pathway. Br J Clin Pharmacol 75(3): 677-696.
- Yoon Y, Song U, Hong SH, Kim JQ (2000) Plasma nitric oxide concentration and nitric oxide synthase gene polymorphism in coronary artery disease. Clin Chem 46(10): 1626-1630.
- Lund, Anders, Shimada, Shigetaka, Shiotani, et al. (2011) Principles and applications of esr spectroscopy. Springer, USA. Facchinetti F, Neri I, Genazzani AR (1996) L-arginine infusion reduces preterm uterine contractions. J Perinat Med 24(3): 383-385.
- Neri I, Mazza V, Galassi MC, Volpe A, Facchinetti F (1996) Effects of L-arginine on utero-placental circulation in growth-retarded fetuses. Acta Obstet Gynecol Scand 75(3): 208-212.
- Meher S, Duley L (2005) Interventions for preventing pre-eclampsia and its consequences: generic protocol. Cochrane Database Systematic Review 2: 1-16.
- Sagol S, Ozkinay E, Ozsener S (1999) Impaired antioxidant activity in women with pre-eclampsia. Int J Gynaecol Obstet 64(2): 121-127.
- Chappell L, Seed PT, Briley AL, Kelly FJ, Lee R, et al. (1999) Effect of antioxidants on the occurrence of pre-eclampsia in women at increased risk: a randomized trial. Lancet 354(9181): 810-816.
- Rees DD, Palmer RM, Moncada S (1989) Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A 86(9): 3375-3378.
- Huang LT, Hsieh CS, Chang KA, Tain YL (2012) Roles of nitric oxide and asymmetric dimethylarginine in pregnancy and fetal programming. Int J Mol Sci 13(11): 14606-14622.
- Visek WJ (1986) Arginine needs, physiological state and usual diets: a re-evaluation. J Nutr 116(1): 36-46.
- Sladek SM, Magness RR, Conrad KP (1997) Nitric oxide and pregnancy. Am J Physiol 272(2 Pt 2): R441–R463.
- Singh S, Singh A, Sharma D, Singh A, Narula MK, et al. (2015) Effect of l-arginine on nitric oxide levels in intrauterine growth restriction and its correlation with fetal outcome. Indian J Clin Biochem 30(3): 298-304.
- Jo T, Takauchi Y, Nakajima Y, Fukami K, Kosaka H, et al. (1998) Maternal or umbilical venous levels of nitrite/nitrate during pregnancy and at delivery. In Vivo 12(5): 523-526.
- Shaamash AH, Elsnosy ED, Makhlouf AM, Zakhari MM, Ibrahim OA, et al. (2000) Maternal and fetal serum nitric oxide (NO) concentrations in normal pregnancy, pre-eclampsia and eclampsia. Int J Gynaecol Obstet 68(3): 207-214.
- Wu G, Morris SM (1998) Arginine metabolism: nitric oxide and beyond. Biochem J 336(Pt 1): 1–17.
- Wu G, Bazer FW, Wallace JM, Spencer TE (2006) Board-invited review intrauterine growth retardation: Implications for the animal sciences. J Anim Sci 84(9): 2316-2337.
- Singh S, Singh A, Sharma D, Singh A, Narula MK, et al. (2015) Effect of l-arginine on nitric oxide levels in intrauterine growth restriction and its correlation with fetal outcome. Indian J Clin Biochem 30(3): 298-304.
- Dorniak-Wall T, Grivell RM, Dekker GA, Hague W, Dodd JM (2014) The role of L-arginine in the prevention and treatment of pre-eclampsia: a systematic review of randomised trials. J Hum Hypertens 28(4): 230- 235.
- Zhu Q, Yue X, Tian QY, Saren G, Wu MH, et al. (2013) Effect of L-arginine supplementation on blood pressure in pregnant women: a meta-analysis of placebo-controlled trials. Am Heart J 162(6): 959-965.
- Dong JY, Qin LQ, Zhang Z, Zhao Y, Wang J, et al. (2011) Effect of oral L-arginine supplementation on blood pressure: a meta-analysis of randomized, double-blind, placebo-controlled trials. Am Heart J 162(6): 959-965.
- Neri I, Jasonni VM, Gori GF, Blasi I, Facchinetti F (2006) Effect of L-arginine on blood pressure in pregnancy-induced hypertension: a randomized placebo-controlled trial. J Matern Fetal Neonatal Med 19(5): 277-281.
- Felipe V, Otilia P, Salvador E, Marco A, Isabel I, et al. (2011) Effect of supplementation during pregnancy with l-arginine and antioxidant vitamins in medical food on pre-eclampsia in high risk population: randomised controlled trial. BMJ 342: d2901.
- Morris N, Eaton BM, Guus Dekker (1996) Nitric oxide, the endothelium, pregnancy and pre-eclampsia. Br J Obstet Gynaecol 103(1): 4-15.
- Conrad KP, Joffe GM, Kruszyna H, Kruszina R, Rochelle LG, et al. (1993) Identification of increased nitric oxide biosynthesis during pregnancy in rats. FASEB J 7(6): 566-571.
- Campese VM, Amar M, Anjali C, Medhat T, Wurgraft A (1997) Effects of L-arginine on systemic and renal haemodynamics in salt-sensitive patients with essential hypertension. J Hum Hypertens 11(8): 527-532.
- Rosano GM, Panina G, Cerquetani E, Leonardo F, Pelliccia F, et al. (1998) L-arginine improves endothelial function in newly diagnosed hypertensives. J Am Coll Cardiol 31: 262.
- Bode-Boger SM, Boger RH, Galland A, Tsikas D, Frolich JC (1998) L-arginine-induced vasodilatation in healthy humans: pharmacokinetic pharmacodynamic relationship. Br J Clin Pharmacol 46(5): 489-497.
- Adams MR, McCredie R, Jessup W, Robinson J, Sullivan D, et al. (1997) Oral L-arginine improves endothelium dependent dilatation and reduces monocyte adhesion to endothelial cells in young men with coronary artery disease. Aterosclerosis 129(2): 261-269.
- Facchinetti F, Neri I, Genazzani AR (1996) L-arginine infusion reduces preterm uterine contractions. J Perinat Med 24(3): 283-285.
- Neri I, Mazza V, Galassi MC, Volpe A, Facchinetti F (1996) Effects of L-arginine on utero-placental circulation in growth- retarded fetuses. Acta Obstet Gynecol Scand 75(3): 208-212.
- Khalil A, Hardman L, O Brien P (2015) The role of arginine, homoarginine and nitric oxide in pregnancy. Amino Acids 47(9): 1715-1727.
- Benedetto C, Marozio L, Neri I, Giarola M, Volpe A, et al. (2000) Increased L-citrulline/L-arginine plasma ratio in severe preeclampsia. Obstet Gynecol 96(3): 395-399.
- Saczko Z, Saczko J, Kulbacka J, Chwiłkowska A, Żorawski K (2009) Pregnancy induced hypertension. Etiopathogenesis. Arterial Hypertens 13: 199-205.
- Ananth CV, Savitz DA, Bowes WA (1995) Hypertensive disorders of pregnancy and stillbirth in North Carolina, 1988 to 1991. Acta Obstet Gynecol Scand 74(10): 788-793.
- Sibai BM, Hauth J, Caritis S, Lindheimer MD, MacPherson C, et al. (2000) Hypertensive disorders in twin versus singleton gestations. National institute of child health and human development network of maternalfetal medicine units. Am J Obstet Gynecol 182(4): 938-994.
- Solomon GC, Carroll JS, Okamura K, Graves SW, Seely EW, et al. (1999) Higher cholesterol and insulin levels in pregnancy are associated with increased risk for pregnancy-induced hypertension. Am J Hypertens 12(3): 276-282.
- Brown MA, Tibben E, Zammit VC, Cario GM, Carlton MA (1995) Nitric oxide excretion in normal and hypertensive pregnancies. Hypertens Pregnancy 14: 319-326.
- Smarason AK, Allman KG, Young D, Redman CW (1997) Elevated levels of serum nitrate, a stable end product of nitric oxide, in women with preeclampsia. Br J Obstet Gynaecol 104(5): 538-543.
- Hata T, Hashimoto M, Kanenishi K, Akiyama M, Yanagihara T, et al. (1999) Maternal circulation nitrite levels are decreased in both normal normotensive pregnancies and pregnancies with preeclampsia preeclampsia. Gynecol Obstet Invest 48(23): 93-97.
- Choi JW, Im MW, Pai SH (2002) Nitric oxide production increases during normal pregnancy and decreases in preeclampsia. Ann Clin Lab Sci 32(3): 257-263.
- Conrad KP, Kerchner LJ, Mosher MD (1999) Plasma and 24-h NOx and cGMP during normal pregnancy and preeclampsia in women on a reduced NOx diet. American Journal of Physiology 277: F48-F57.
- Mata-Greenwood E, Chen D (2008) Racial differences in nitric oxidedependent vasorelaxation. Reprod Sci 15(1): 9-25.
- Brewster LM, Taherzadeh Z, Volger S, Clark JF, Rolf T, et al. (2010) Ethnic differences in resistance artery contractility of normotensive pregnant women. Am J Physiol Heart Circ Physiol 299(2): H431-H436.
- Fallah F, Pourabbas A, Delpisheh A, Veisani Y, Shadnoush M (2013) Effects of nutrition education on levels of nutritional awareness of pregnant women in western Iran. Int J Endocrinol Metabo 11(3): 175-178.
- Johnson K (2000) A study of nutritional knowledge and supplement use in pregnant women. J Human Nutr Dietetics 13(5): 363-371.
- Verbeke W, De Bourdeaudhuij I (2007) Dietary behaviour of pregnant versus non-pregnant women. Appetite 48(1): 78-86.
© 2018 Osaro Erhabor. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






