- Submissions

Full Text
COJ Electronics & Communications
Effect of Chemical Variables on Laminar Burning Velocity of Liquid Fuels and Gases
Khudhair O1 and Obayes HK2*
1Al-Furat Al-Awsat Technical University, Iraq
2Ministry of Education, Iraq
*Corresponding author: Hayder Khudhair Obayes, Ministry of Education, Iraq
Submission: April 12, 2020;Published: May 17, 2021

ISSN 2640-9739Volume2 Issue1
Abstract
Laminar burning velocity is one of the important aspects of fuel combustion characteristic. It affects combustion system design and performance. A lot of researches work related to laminar burning velocity had been carried out, but these works were speared in different journals and conference proceeding. The aim of this study is to show the most chemical variables that effect on this important combustion property. These variables include mixture strength, molecular structure, inert additives and effect of oxygen as oxidizer.
Keywords: Burning velocity; Bunsen burner; Internal combustion engines
Introduction
Laminar burning velocity is one of the fundamental properties of a reacting premixed mixture and its reliable data are constantly needed for combustion applications. So far, several techniques for measuring the one-dimensional laminar burning velocity have been used for a wide range of temperatures, pressures and fuels .Some of these techniques flat or curved flames in stagnation flow, propagating spherical flames in combustion vessel and flat flames stabilized on burner. With all these measurement techniques proper care should be taken to remove the effect of flame stretch either during experimentation or through further data processing.
Related Work
The most relevant definition of flames is “visible chemical component undergoing highly exothermic chemical reaction takes place in a small zone with the evolution of heat”. The flame speed is the measured rate of expansion of the flame front in a combustion reaction [1]. Flames can be produced in two radically different ways depending upon how the reactants are brought together, premixed and non-premixed. In the premixed flame, the oxidizer has been mixed with the fuel before it reaches the flame front. The reaction creates a thin flame front as all of the reactants are readily available. The pre-mixed flammability limits for most hydrocarbon fuels are 0.6<ϕ<3 [2]. In a diffusion flame (non-premixed), the reactants are initially separated, and the reaction occurs only at the interface between the fuel and oxidizer, where mixing and reaction both take place. It can also be defined as the flame in which the oxidizer combines with the fuel by diffusion. As a result, the flame speed is limited by the rate of diffusion. Diffusion flames tend to burn slower and to produce more soot than premixed flames because there may not be sufficient oxidizer for the reaction to complete [1].
Chemical Variables
Effect of mixture strength (Φ)
Mixture strength or equivalence ratio (Φ) is defined as the ratio of the actual to the stoichiometric air-fuel ratios. It is generally acceptable to assume that a mixture with a maximum flame temperature is also has a maximum burning velocity [3]. It is clear that very lean and very rich mixtures fail to support a propagatable flame because there is too little fuel or oxidant to maintain a steady deflagration wave. Thus, there exist upper and lower flammability limits [4,5] measured the laminar burning velocity of H2-natural gas/air mixture in a constant volume bomb at temperature (298K), pressure (1atm.) and equivalence ratios (ϕ) from 0.6 to 1.4. The results showed that for lean and rich mixture combustion, there exists a linear correlation between flame radius and time. Combustion of stoichiometric mixture demonstrated the linear relationship between flame radius and time for natural gas/air, hydrogen/air, and natural gas/hydrogen/air flames. Flame instability increased with the increase of hydrogen fractions in the mixture. Based on the experimental data, a formula for calculating the laminar burning velocity of natural gas/hydrogen/air flames was proposed. Liao et al. [6] carried out a study on determination of the laminar burning velocities for mixtures of ethanol and air at elevated temperatures. They measured the laminar burning velocities for ethanol-air premixed mixtures at various initial temperature and equivalence ratio. The flames are analyzed to estimate flame size, consequently, the flame speeds are derived from the variations of the flame size against the time elapsed. They also studied the effects of equivalence ratio, initial temperature, and pressure on the laminar flame propagation. They studied the premixed laminar combustion of ethanol-air mixture experimentally in a closed combustion bomb and found that the laminar burning velocity was 58.3cm/s at initial pressure of 0.1MPa. and temperature of 358K. Xuan et al. [7] carried out a study on measurements of laminar burning velocities and flame stability analysis for dissociated methanol-airdiluents mixtures at elevated initial temperatures and pressures and equivalence ratios. In this study the laminar burning velocities and Markstein lengths for the dissociated methanol-air-diluent mixtures were measured. It was found that the peak laminar burning velocity occurs at equivalence ratio of 1.8. The Markstein length decreases with the increase in initial temperature and initial pressure. Measurements of laminar burning velocities and flame stability analyses were conducted using the outwardly propagating spherical laminar premixed flame. Shuang et al. [8] carried out a study on Laminar burning velocities and Markstein lengths of premixed methane/air flames near the lean flammability limit. Outwardly propagating spherical flames were employed to assess the sensitivities of the laminar burning velocity to flame stretch, represented by Markstein lengths, and the fundamental laminar burning velocities of unstretched flames. Resulting data were reported for methane/air mixtures at ambient temperature and pressure, over the specific range of equivalence ratio that extended from 0.512 to 0.601. Furthermore, the burning velocities were predicted by three chemical reaction mechanisms. Additional results of this investigation were derived for the overall activation energy and corresponding Zeldovich numbers, and the variation of the global flame Lewis numbers with equivalence ratio.
Effect of fuel molecular structure
Effect of fuel structure (number of carbon atoms) on the laminar burning velocity had been studied by Kuo KK et al. [9,10]. They found that the laminar burning velocity decreases with the increase in number of carbon atoms. Bradley et al. [11] found that for lean mixtures of the liquid fuel group, the burning velocity varied approximately linearly with the heat of reaction per kilomole of the mixture. They were able to plot different lines for different pressures and functional groups. Marshall et al. [12] investigated LBV of n-heptane, iso-octane, toluene, ethylbenzene and ethanol over a wide range of initial pressures, temperatures and equivalence ratios, along with tests using combustion residuals.
Effect of inert additives
Many investigators studied the effects of the inert additives to combustible mixture such as (CO2 , N2 , He and Ar), on their burning velocities. Erjiang et al. [13] studied the effect of diluents on the laminar burning velocity of the premixed methane-airdiluent flames numerically. The mechanisms of diluent, thermal diffusion and chemical effects of diluent on the burning velocity were analyzed at different dilution ratios for different diluents. Results showed that the burning velocity was decreased in the order from helium, argon, nitrogen and carbon dioxide. Benedicte et al. [14] investigated effects of dilution on premixed methane/ air combustion through experiments and numerical simulations on laminar burning velocities. Laminar burning velocities were determined for several diluents (nitrogen, carbon dioxide, water vapor, a mixture of the three previous gases representative of exhaust gases, hilum and argon) and for different dilution percentages. Excellent agreements between experimental data and computed results are obtained.
Effect of oxygen as oxidizer
Oxygen is one of the more important factors that affecting the measurements and values of laminar flame speed and burning velocity. Han et al. [15] and Oh & Noh [16], determined the burning velocities of methane air and methane oxygen enriched flame for various equivalence ratios by an analysis of the Schlieren image. Modified reaction mechanism showed a good agreement for predicting the burning velocity in methane /oxygen enriched flame as well as methane/air flame. Mazas et al. [17] investigated effects of water vapor addition on premixed methane oxygen-enhanced combustion through experiments and numerical simulations on laminar burning velocities of CH4/O2/N2/H2O (v) mixtures. The tests were carried out at atmospheric pressure and fixed inlet temperature Tu=373K. The mixture equivalence ratio was varied from 0.6 to 1.5. The oxygen enrichment ratio in the oxidizer, defined as O2/(O2+N2) (mol.), is varied from 0.21 (air) to 1.0 (pure oxygen). The equivalence ratio ranges from 0.5 to 1.5 and the steam molar fraction in the reactive mixture is varied from 0 to 0.50. Experimental data yield a linear decrease of the laminar burning velocity when the water vapor molar fraction was increased. For an oxygen enrichment ratio in the oxidizer O2 (O2+N2) (mol.) equal to 0.5, this decrease was found to be independent of the equivalence ratio and a correlation was proposed to decrease the effects of water vapor on the laminar burning velocity. Hernando A et al. [18] studied the performance of laminar burning velocity of a mixture of H2, CO and N2 (20:20:60 vol.% ) using air enriched with oxygen as the oxidizer, varying the oxygen content from 21% up to 35% for different equivalence ratios. The laminar burning velocity increased with the concentration of the oxygen in the mixture due to the increase of the reaction rate for a stoichiometric mixture, the laminar burning velocity increased by almost 25% with an increment of 4% of oxygen in the oxidant.
Fuel type
The molar mass of the fuel has a weak effect on the burning velocity since the vast majority of the mixture is air. Far more significant is the enthalpy of combustion of the fuel since this affects the adiabatic flame temperature where the temperature has the greatest effect on burning velocity. The kinetics of the oxidation reactions are also important and can explain why some fuels with similar adiabatic flame temperatures have different burning velocity [19].
Experimental Results
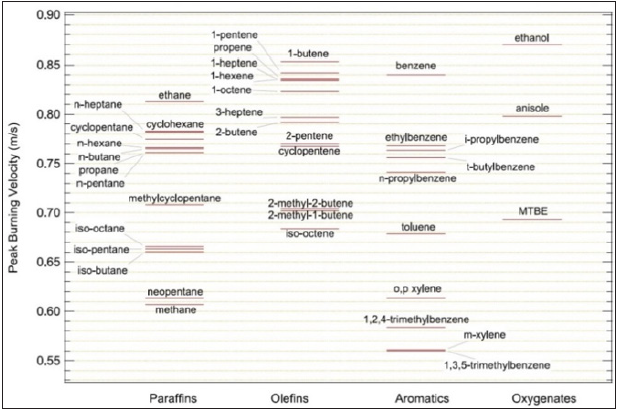
Figure 1 studied the effect of temperature on the burning velocity of hydrogen flames for different equivalence ratios and validated the mechanism with many researches and found that the slightly richer than stoichiometric is the region of the maximum LBV. Figure 2 tested a 45 type of hydrocarbon fuels using a constant volume bomb, but only at an initial temperature of (450K) and an initial pressure of (304kPa) and also the burning velocity of alkanes is higher than others and the branches of alkane will decrease the flame speed.
Figure 1: LBV of H2/air flames at standard conditions for ϕ=0.25–7 (left) and ϕ<1 (right).

Figure 2: Peak-burning velocities for chemicals of different families.
Conclusion
With the increase of equivalence ratio, laminar burning velocity increases in the case of fuel-lean mixture combustion and decreases in the case of fuel-rich mixture combustion. Laminar burning velocity decreases with the increase of dilution ratio, and the position of peak value of laminar burning velocity moves slightly to the lean mixture direction with the increase of dilution ratio. With the increase of equivalence ratio, the flame stability is increased. Markstein length increases monotonously with the increase of equivalence ratio and it slightly decreases with the increase of dilution ratio. Various diluents (N2, Ar and He) highlight effects of transport and chemistry on preferential-diffusion/stretch interactions. Replacing nitrogen with argon yielded larger laminar burning velocities due to increased flame temperatures but had a relatively small effect on Markstein numbers due to nearly same transport properties for the two diluents. Replacing nitrogen with helium, however, yielded a larger laminar burning velocities due to both increased flame temperatures and transport rates, and also stabilized lean flames by enhancing the diffusion of heat.
References
- Taylor, Simon Crispin (1991) Burning velocity and the influence of flame stretch. Department of Fuel and Energy, University of Leeds, UK.
- Strahle WC (1993) An Introduction to combustion science and technology. (1st edn), Gordon and Breach Science Publishers, UK, pp. 1-192.
- Barnard JA, Bradley JN (1984) Flame and combustion. (1985th edn), Springer Publishers, USA, pp. 1-308.
- Turns RS (2000) An introduction to combustion. (2nd edn), McGraw-Hill Higher Education, USA, pp. 1-704.
- Zuohua H, Yong Z, Ke Z, Bing L, Qian W, et al. (2006) Measurements of laminar burning velocities for natural gas-hydrogen-air mixtures. Combustion and Flame 146(1-2): 302-311.
- Liao SY, Jiang DM, Huang ZH, Zeng K, Cheng Q (2007) Determination of the laminar burning velocities for mixtures of ethanol and air at elevated temperatures. Applied Thermal Engineering 27(2-3): 374-380.
- Zhang X, Huang Z, Zhang Z, Zheng J, Wu Yu, et al. (2009) Measurements of laminar burning velocities and flame stability analysis for dissociated methanol-air-diluents mixtures at elevated temperatures and pressures. International Journal of Hydrogen Energy 34(11): 4862-4875.
- Shuang-Feng W, Hai Z, Jarosinski J, Gorczakowski A, Podfilipski J (2010) Laminar burning velocities and Markstein lengths of premixed methane/air flames near the lean flammability limit in microgravity. Combustion and Flame 157(4): 667-675.
- Kuo KK (1986) Principle of combustion. (2nd edn), John Wiley and Sons, USA.
- Gibbs GJ, Calcote HF (1959) Effect of molecular structure on burning velocity. Journal of Chemical and Engineering Data 4(3): 226-237.
- Bradley D, Habika SED, El-Sherif SA (1991) A generalization of laminar burning velocities and volumetric heat release rates. Combustion and Flame 87(3-4): 336-345.
- Marshall SP, Taylor S, Stone CR, Davies TJ, Cracknell RF (2011) Laminar burning velocity measurements of liquid fuels at elevated pressures and temperatures with combustion residuals. Combustion and Flame 158(10): 1920-1932.
- Erjiang Hu, Huang Z, He J, Zheng J, Miao H (2009) Measurements of laminar burning velocities and onset of cellular instabilities of methane-hydrogen-air flames at elevated pressures and temperatures. International Journal of Hydrogen Energy 34(13): 5574-5584.
- Galmiche B, Halter F, Foucher F (2012) Effects of high pressure, high temperature and dilution on laminar burning velocities and Markstein lengths of iso-octane/air mixtures. Combustion and Flame 159(11): 3286-3299.
- Han JW, Lee CE, Kum SM, Hwang YS (2007) Study of the improvement of chemical reaction mechanism of methane based on the laminar burning velocities in OEC. Energy and Fuels 21(6): 3202-3207.
- Oh J, Noh D (2012) Laminar burning velocity of oxy-methane flames in atmospheric conditions. Energy 45(1): 69-75.
- Mazas AN (2010) Study of oxygen-enriched premixed flames: Effects of water and carbon dioxide addition. Ph.D. Thesis, France.
- Yepes HA, Amell AA (2013) Laminar burning velocity with oxygen-enriched air of syngas produced from biomass gasification. Journal of Hydrogen Energy 38(18): 7519-7527.
- Marshall, Stephen P (2010) Measuring laminar burning velocities. University of Oxford, UK.
© 2020 Obayes HK. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






