- Submissions

Full Text
COJ Biomedical Science & Research
Early Diagnosis of Anti-N-Methyl-D-Aspartate Receptor (Anti-NMDAR) Encephalitis with Predominant Cognitive Symptoms: A Case Study
Natalya Yarovinsky*, Tali Fisher, Yarden Avnor, Rachel Ben Hayun, Rafi Hadad and Judith Aharon Peretz
Rambam Health Care Campus, Israel
*Corresponding author: Natalya Yarovinsky, Rambam Health Care Campus, Haaliya Hashniya St 8, Haifa, 3109601, Israel
Submission: March 13, 2023; Published: April 04, 2023

Volume2 Issue3April , 2023
Abstract
Anti-NMDAR encephalitis is an autoimmune syndrome primarily affecting young women and often associated with bilateral ovarian teratoma. As the hallmark clinical manifestations of this condition are neuropsychiatric symptoms, the diagnosis is often delayed, potentially leading to irreversible brain damage. We present the unique case of a 30-year-old female with an acute emergence of memory impairment ultimately diagnosed as caused by anti-NMDAR encephalitis and highlight the potential use of cognitive assessment as a sensitive tool for analyzing the clinical course and prognosis of autoimmune mediated encephalitis.
Keywords:Anti-NMDAR autoimmune encephalitis; Recognition; Familiarity; Recollection
Introduction
The most common type of autoimmune encephalitis, Anti-N-methyl-D-aspartate receptor (anti-NMDAR) encephalitis [1] was first described by Dalmau et al. [2] and has been shown to frequently affect young women with ovarian teratomas [3,4] While this autoimmune encephalitis is treatable, it can be life-threatening if not caught early on. Since the most prominent initial presenting symptoms are neuropsychiatric [5,6], early diagnosis is often difficult. Additional clinical presentations may include epileptic seizures, behavioral and consciousness changes, and cognitive decline [7]. This case study demonstrates the complexity of diagnosis and differential diagnosis.
Case Presentation
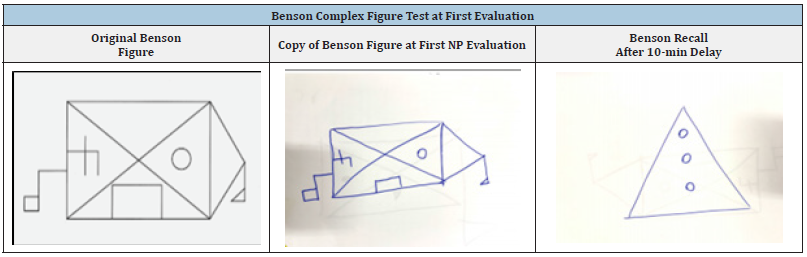
L.L. is a 30- year-old, female, social worker. Her past medical and psychiatric history was uneventful except for the diagnosis of attention deficit hyperactivity disorder at the age of 10. L.L. was hospitalized in the neurology department due to her complaints of elevated anxiety, clouded and confused thinking, “blackouts” and mood changes. Her husband reported that during the two weeks prior to her hospitalization, L.L. seemed very nervous, unfocused and unable to recall events throughout the day. He further explained that L.L. was behaving oddly, such as asking the same question over and over again and needing frequent reminders in carrying out basic daily tasks such as teeth brushing and showering. Eight months before her admission, during the COVID-19 pandemic, L.L. had a normal childbirth delivery and reported feeling more lonely, sad and anxious than usual. Due to her change in mood, L.L. began meeting with a psychologist regularly. L.L remembers retrospectively speaking coherently during this time, although her psychologist noticed a change in her train of thought and attributed these changes to possibly postpartum depression. Throughout the neuropsychological (NP) evaluation examination, L.L appeared very stressed. She cried and repeatedly asked if she was going to die. Nevertheless, a formal testing measure (TOMM) indicated normal cooperation indices. The NP exam revealed preserved performance in language functions and visual perception. The immediate recall of information was within the norm. A noticeable impairment was observed in L.L’s episodic memory when presented with verbal and visual material (Benson test, RAVLT, Logical memory). However, when L.L performed NP tasks relying on recognition (RAVLT, TOMM), her performance partially improved, as seen in Tables 1&2.
Table 1:L.L’s neuropsychological exam scores at two time points: (1) upon her initial hospitalization and (2) after treatment.
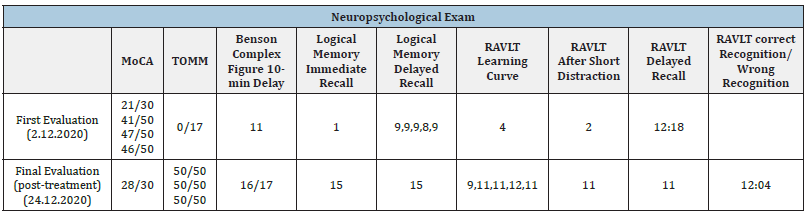
Table 2:L.L’s copy and recall during the Benson Complex Figure Test upon first evaluation.
The diagnostic work up
The blood count, electrolytes, glucose, renal, liver, thyroid function tests, serum vitamins B12 and folate, lipid profile, autoimmune serology antibodies, neoplastic markers, serology for CMV, EBV, WNV, COVID-19, HIV, syphilis and Mycoplasma and MRI of the brain were normal. CSF analysis revealed 4 lymphocytes and protein and glucose levels were normal. Cytology, virology (HSV, HZV, and enterovirus) and bacteriology (syphilis, cryptococcal antigen) of CSF were negative. The oligoclonal band was negative. EEG depicted bitemporal epileptiform discharges as more prominent on the left. A panel of paraneoplastic and non-paraneoplastic antibodies in blood and CSF revealed positive antibodies for NMDAR. Gynecologic ultrasonography revealed polycystic ovaries with bilateral dermoid cysts up to 15mm with hyperechoic areas.
Diagnosis & treatment
L.L. was diagnosed with anti-NMDAR autoimmune encephalitis. Treatment included IV Methylprednisolone (4 grams for 4 days) followed by plasmapheresis (5 alternate-day treatments) and bilateral dermoid cystectomy.
Discussion
We present the case study of a young woman with anti-NMDA receptor encephalitis presenting with behavioral changes and memory impairment. The NP testing was an integral part of the anti-NMDA receptor encephalitis diagnosis. Furthermore, by using NP testing, we were able to realize that while L.L’s ability to acquire and retrieve new information was impaired, her ability to perform recognition tasks relying on familiarity memory processes were relatively intact. We believe that this finding in the NP evaluation can help shed light on the understanding of how memory processes work. For this reason, we would now like to elaborate on the cognitive processes of recognition, familiarity, and recollection. It is well accepted that recognition memory reflects the contribution of two separable memory retrieval processes, recollection, and familiarity. Recollection is a specific type of memory process that occurs when a “test stimulus prompts retrieval of what one was doing, thinking, or feeling when the stimulus was previously encoded” [8]. “Familiarity refers to knowing that an item was presented earlier, with no additional contextual information retrieved” [9]. It is assumed that in familiarity, the process of remembering relies on the perceived memory strength of the recognized stimulus [10].
The Dual-Process theories of recognition suggest that the two processes, recognition and familiarity, depend on separate neural substrates [11-25]. Recollection mainly dependent on the hippocampus, while familiarity mainly depending on outside the hippocampus substrates [26,27]. A different explanation to the recollection-familiarity discrepancy is offered by The Single- Process View, which suggests that the neural substrates for recollection and familiarity are nearly the same, differing only in their degree of involvement and the strength of memory encoding single-process view [28-32].
Since L.L’s cognitive profile in the NP assessment revealed a specific deficit in recollection memory (RAVLT, Logical memory, and Benson Complex Figure) and intact familiarity (Test of Malingering – TOMM test, a 50 item, two-alternative forced-choice test), we believe that NMDAR encephalitis could serve as a suitable clinical platform for future anatomo-functional research in examining “single” or “dual-process” recognition-recollection memory process.
Conclusion
LL’s case demonstrates the value of incorporating early NP testing and cognitive functioning exams in neurological evaluations. In addition, to the best of our knowledge, this is the first time that the cognitive processes of familiarity and recollection are examined in a case of anti-NMDA receptor encephalitis.
References
- Dalmau J, Graus F (2018) Antibody-mediated encephalitis. N Engl J Med 378(9): 840-851.
- Dalmau J, Tüzün E, Wu H, Masjuan J, Rossi JE, et al. (2007) Paraneoplastic Anti-N-methyl-D-aspartate receptor encephalitis associated with ovarian teratoma. Ann Neurol 61(1): 25-36.
- Gable MS, Sheriff H, Dalmau J, Tilley DH, Glaser CA (2012) The frequency of autoimmune N-methyl-D-aspartate receptor encephalitis surpasses that of individual viral etiologies in young individuals enrolled in the California encephalitis project. Clinical Infectious Diseases 54(7): 899-904.
- Tanguturi YC, Cundiff AW, Fuchs C (2019) Anti-N-Methyl D-aspartate receptor encephalitis and electroconvulsive therapy: Literature review and future directions. Child and Adolescent Psychiatric Clinics of North America 28(1): 79-89.
- Elamin M, Farid F, Dana M, Alam M, Mazin A, et al. (2018) Psychiatric manifestation of anti-NMDA receptor autoimmune encephalitis. Dubai Medical Journal 1(1-4): 26-28.
- Nichols TA (2016) Anti-NMDA receptor encephalitis: An emerging differential diagnosis in the psychiatric community. The Mental Health Clinician 6(6): 297-303.
- Scalici F, Caltagirone C, Carlesimo GA (2017) The contribution of different prefrontal cortex regions to recollection and familiarity: A review of fMRI data. Neuroscience and Biobehavioral Reviews 83: 240-251.
- Kim H (2021) Imaging recollection, familiarity, and novelty in the frontoparietal control and default mode networks and the anterior-posterior medial temporal lobe: An integrated view and meta-analysis. Neuroscience and Biobehavioral Reviews 126: 491- 508.
- Montaldi D, Mayes AR (2010) The role of recollection and familiarity in the functional differentiation of the medial temporal lobes. Hippocampus 20(11): 1291-1314.
- Brown MW, Aggleton JP (2001) Recognition memory: What are the roles of the perirhinal cortex and hippocampus? Nature Reviews Neuroscience 2(1): 51-61.
- Eichenbaum H, Yonelinas AP, Ranganath C (2007) The medial temporal lobe and recognition memory. Annu Rev Neuroscience 30: 123-152.
- Yonelinas AP, Aly M, Wang WC, Koen JD (2010) Recollection and familiarity: Examining controversial assumptions and new directions. Hippocampus 20(11): 1178-1194.
- Atkinson RC, Juola JF (1974) Search and decision processes in recognition memory. In: Krantz DH, Atkinson RC, Luce RD, Suppes P (Eds.), Contemporary Developments in Mathematical Psychology: I. Learning, Memory and Thinking. W. H. Freeman, USA.
- Mandler G (1980) Recognizing: The judgment of previous occurrence. Psychological Review 87(3): 252-271.
- Jacoby LL (1991) A process dissociation framework: Separating automatic from intentional uses of memory. Journal of Memory and Language 30(5): 513-541.
- Yonelinas AP (1994) Receiver-operating characteristics in recognition memory: Evidence for a dual-process model. Journal of Experimental Psychology Learning, Memory, and Cognition 20(6): 1341-1354.
- Aggleton JP, Brown MW (1999) Episodic memory, amnesia and the hippocampal-anterior thalamic axis. Behavioral and Brain Sciences 22(3): 425-444.
- Reder LM, Nhouyvanisvong A, Schunn CD, Ayers MS, Angstadt P, et al. (2000) A mechanistic account of the mirror effect for word frequency: A computational model of remember-know judgments in a continuous recognition paradigm. Journal of Experimental Psychology: Learning Memory and Cognition 26(2): 294-320.
- Kelley R, Wixted JT (2001) On the nature of associative information in recognition memory. Journal of Experimental Psychology Learning Memory and Cognition 27(3): 701-722.
- Rotello CM, Macmillan NA, Reeder JA (2004) Sum-difference theory of remembering and knowing: A two-dimensional signal-detection model. Psychological Review 111(3): 588-616.
- Yonelinas AP (2001) Consciousness, control, and confidence: The 3 Cs of recognition memory. Journal of Experimental Psychology: General 130(3): 361-379.
- Yonelinas AP (2002) The nature of recollection and familiarity: A review of 30 years of research. Journal of Memory and Language 46(3): 441-517.
- Squire LR, Wixted JT, Clark RE (2007) Recognition memory and the medial temporal lobe: A new perspective. Nature Reviews Neuroscience 8(11): 872-883.
- Wais PE, Rubens MT, Boccanfuso J, Gazzaley A (2010) Neural mechanisms underlying the impact of visual distraction on retrieval of long-term memory. Journal of Neuroscience 30(25): 8541-8550.
- Wixted JT, Mickes L (2010) A continuous dual-process model of remember/know judgments. Psychological Review 117(4): 1025-1054.
- Aggleton JP, Brown MW (2006) Interleaving brain systems for episodic and recognition memory. Trends in Cognitive Sciences 10(10): 455-463.
- Dalmau J, Lancaster E, Martinez HE, Rosenfeld MR, Balice GR (2011) Clinical experience and laboratory investigations in patients with anti-NMDAR encephalitis. Lancet Neurology 10(1): 63-74.
- Diana RA, Yonelinas AP, Ranganath C (2007) Imaging recollection and familiarity in the medial temporal lobe: A three-component model. Trends in Cognitive Sciences 11(9): 379-386.
- Heine J, Kopp UA, Klag J, Ploner CJ, Prüss H, et al. (2021) Long-term cognitive outcome in anti-N-methyl-D-aspartate receptor encephalitis. Ann Neurol 90(6): 949-961.
- Huang Q, Xie Y, Hu Z, Tang X (2020) Anti-N-methyl-D-aspartate receptor encephalitis: A review of pathogenic mechanisms, treatment, prognosis. Brain Research 1727: 146549.
- Nasreddine ZS, Phillips NA, Bédirian V, Charbonneau S, Whitehead V, et al. (2005) The montreal cognitive assessment, MoCA: A brief screening tool for mild cognitive impairment. Journal of the American Geriatrics Society 53(4): 695-699.
- Tombaugh TN (1997) The Test of Memory Malingering (TOMM): Normative data from cognitively intact and cognitively impaired individuals. Psychological Assessment 9(3): 260-268.
© 2023 Natalya Yarovinsky. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






