- Submissions

Full Text
COJ Biomedical Science & Research
Dry Blood Spots for Monitoring SARS-CoV-2 IgG Antibody Titres-a Pilot Study
Franz Tatzber1, Ulrike Resch2, Matthias Kuper3, Chittaranjan Bhaduri4, Willibald Wonisch1* and Gerhard Cvirn5
1Omnignostica Ltd, 3421 Höflein at the Danube, Austria
2Department of Vascular Biology and Thrombosis Research, Medical University of Vienna, Austria
3LDN Ltd, Nordhorn, Germany
4RD-Ratio Diagnostics, Frankfurt, Germany
5Otto Loewi Research Center, Division of Physiological Chemistry, Medical University of Graz, Neue Stiftingtalstraße 6 HBK M1/D3, 8010 Graz, Austria
*Corresponding author: Willibald Wonisch, Omnignostica Ltd, A-3421 Höflein at the Danube, Austria
Submission: November 9, 2022; Published: November 28, 2022

Volume2 Issue2November , 2022
Abstract
Background: Activating the specific immune system by vaccination against COVID-19 is crucial to strive
against this pandemic. Constant and efficient monitoring of IgG antibody titres is valuable for evaluating
immunizations. However, serum or plasma samples, commonly used in follow-up active immunizations,
require a visit to the doctor´s office to be handled as a biohazard and need cold chain transportation and
storage.
Methods: In contrast, dry blood spots can be self-collected and represent a minimal biohazard potential,
even if they contain human pathogenic viruses. Blood sampling was performed on 12 volunteers (8
females) for six months after vaccination. Whole blood drops from a finger pulp were applied on filter
paper cards or collected in a Microvette for serum samples. Analysis was performed with a COVID-19
enzyme-linked immunosorbent assay.
Results: The comparison between dry blood spots and serum samples yielded similar results for SARSCoV-
2 IgG antibody titres. Daily blood sampling from the same subject revealed an initial boost in the
generation of antibody titres that descended after a peak level. Monitoring convalesced subjects after
a COVID-19 infection resulted in SARS-CoV-2 IgG antibody-titres of the same magnitude or higher
as vaccinated patients with at least three immunizations. An unvaccinated subject without infection
showed SARS-CoV-2 IgG antibody constantly titres below the cutoff level of the assay during the whole
observation period.
Conclusion: These results indicate that monitoring SARS-CoV-2 IgG antibody titres from dry blood spot
excisions is a user-friendly and reliable technique with a minimal biohazard risk regarding the transport
and analysis of these samples.
Keywords:Dry blood cards; COVID-19; Antibody titre; Populations immunity; SARS-CoV-2; Serum
Introduction
The benefits of monitoring specific IgG molecules in pandemic and endemic events are generally accepted [1]. The median seroconversion time in patients with SARS-CoV-2 infection was ascertained on days 12 for immunoglobulin M (IgM) and 14 for IgG antibodies. Less than 40 per cent of patients developed antibody titres within the first week, followed by a rapid increase two weeks after onset. High antibody titres were also allied with a worse clinical classification. Therefore, antibody measurement provides clinical information in patients infected with SARS-CoV-2 [2] and also offers valuable information in the follow-up of vaccinated subjects concerning their immune status. The protective capacity and duration of humoral SARS-CoV-2 immunity might provide evidence about the efficacy of SARS-CoV-2 vaccines and is, therefore, decisive for managing the pandemic and public health decisions [3]. Detection of antigens with Real-Time Polymerase Chain Reaction (RT-PCR) tests is the gold standard for monitoring the presence of the virus, among other diagnostic techniques. Thus, it is a valuable tool in acute infections but is ineffective in discriminating between live versus inactive viruses and measuring the success of active immunizations or the hazard of anaphylactic reactions [4]. Several shortcomings were reported, including falsepositive, false-negative rates, extraction, time and labour, highly sophisticated equipment and dependence on the method [5-7]. In addition, significant SARS-CoV-2 antibody titres up to 64% were observed among SARS-CoV-2 PCR-negative subjects. Thus, continuous serological surveillance for transmission dynamics is necessary to support public health authorities, as mentioned by these authors [8]. Moreover, people who did not develop sufficient amounts of anti-COVID-19 IgG antibodies [9, 10], may receive alternatives, e.g., a booster vaccination [11], to push immunization at earlier time points if these insufficient IgG levels are detected precociously. On the other hand, serum or plasma samples collected for IgG measurement may contain pathogenic virus loads and therefore pose a biohazard potential. Furthermore, it is also well known that certain religions, e.g., Mormons, refuse a blood draw from a peripheral vein. From these considerations, finding alternative sample systems from which SARS-CoV-2 IgG antibody titres can be detected reliably is desirable. Dry Blood Spot (DBS) sampling may be a promising alternative blood collection system. Its main advantages are self-collection through a fingerstick at home, and after drying, the samples pose minimal biohazard potential [12]. Further advantages of a DBS sampling system are facilitated sample management and storage, as no freezing or refrigeration are required for storing or shipping such samples. In a recent publication by Itell et al. [13], DBS reflected plasma antibody binding by ELISA and comprised SARS-CoV-2 neutralization titres, which correlated with plasma. In addition, DBS are applicable for polymerase chain reaction (PCR) and micro assays [12,14] besides determining antibody titres that are comparable with a liquid specimen [15]. From these considerations, this study aimed to evaluate a DBS sampling system to screen SARS-CoV-2 IgG antibody titres, including an adjustment for the enzyme-linked immunosorbent assay. We hypothesized that SARS-CoV-2 antibody titres from DBS correlate with serum antibody titres. Moreover, we determined the daily increase of SARS-CoV-2 IgG antibodies for 69 days in a male volunteer after the initial immunization. In addition, the antibody titres of different age groups and the longevity of vaccine-elicited responses were investigated. We hypothesized that DBS is a cheap, safe and feasible blood sampling device to monitor the populations immunity during the pandemic, mainly to prevent the pathogen´s spread.
Methods
Study participants
Volunteers (n = 12, males 4, females 8, aged 25-89 years) were divided in unvaccinated (n = 1), vaccinated (n = 8), and vaccinated plus convalesced (n = 3).
Blood sampling
Whole blood was obtained from a finger pulp by pricking a hyperemic fingertip with a lancet (Accu-Check, Softclix, Roche Diagnostics, Basel, Switzerland). The blood drops were resorbed onto the filter of Dry Blood Cards (DBS) collecting devices (LDN, Labor Diagnostic Nord, Nordhorn, Germany) so that at least 5 mm in diameter were soaked and allowed to dry at room temperature (22 °C +/- 2 °C). The storage was at ambient temperature until use, but no longer than 2 weeks. Circle-shaped excisions from dry blood filter paper were obtained through a punch press with a diameter of 2-5 mm. After preliminary examinations, the best results were achieved using dry blood spots of 3mm in diameter. In the case of serum samples, we took capillary blood in equal measure and collected the blood into a 200μL Microvette (Sarstedt). The first drop of capillary blood was discarded. After coagulation (30 min) at room temperature, the samples were centrifuged at 3000 rpm for 10 min in an Eppendorf centrifuge. Serum samples were stored at -20 °C until use, but no longer than 2 weeks.
COVID-19 IgG enzyme-linked immunosorbent assay (ELISA)
SARS-CoV-2 IgG antibody titres were determined with an ELISA purchased from RD Ratio-Diagnostics (Frankfurt, Germany). In the case of DBS, a single modification was necessary according to the operating procedure. In brief, the first incubation with diluted serum samples (30 minutes at 37 °C) was substituted by elution of the blood-sodden filters (3mm in diameter) in the wells of the microtitration plates for 120 minutes at room temperature on a rocking device with 100 μl assay buffer in each well. Filters were removed within the first washing step. The other tasks were performed according to the procedure manual. All analyses were performed in duplicates, and plates were analyzed photometrically on a microplate reader (Epoch 2, BioTek, California, USA) at a wavelength of 450nm. Results were expressed as Binding-Antibody Units per millilitre (BAU/mL).
Data generation and processing
Statistical procedures were carried out using either Microsoft Excel (Redmond, Washington, USA) or SPSS2 software (IBM, Armonk, New York, USA).
Results
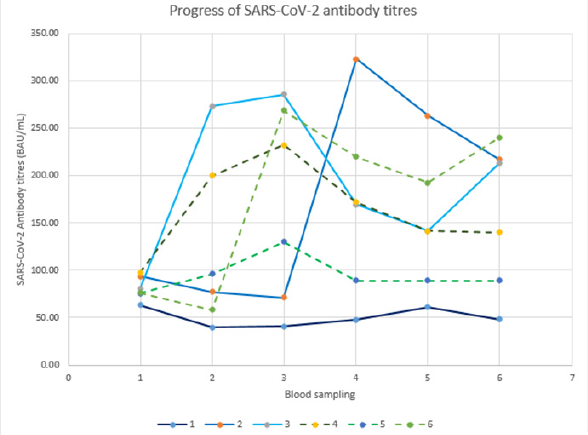
The conformity between serum samples and dry blood spots is indicated in Table 1 from three female volunteers. DBS-excisions of 3mm in diameter gave almost identical results as 1:100 diluted serum samples following the procedure manual of the ELISA. Therefore, it can be stated that SARS-CoV-2 IgG titres from 3mm DBS excisions are consistent with those obtained from serum samples. However, it should be noted that it was impossible to include the WHO standard in the DBS evaluation. The SARS-CoV-2 IgG titres from a single male volunteer (age=67 years) correlated with the time after the initial immunization with a correlation coefficient of (r=0.9179), as shown in (Figure 1). The highest positivity for IgG antibodies against SARS-CoV-2 was reported between days 31- 60 post symptom onset [16,17]. In the same line, we started IgG antibody determination daily from day 47 to 116. A comparison of healthy versus infected volunteers after booster vaccination is given in (Figure 2). Volunteers V1vac (female, 72 years-3x Comirnaty® (Pfizer/BioNTech)) and V2vac (male, 67 years - 2x Vaxzevria® (AstraZeneca); 1x Comirnaty® (Pfizer/BioNTech)) received booster vaccinations and kept well and fit. Volunteers V3vac-con (female, 59 years - 2x Vaxzevria® (AstraZeneca); 1x Comirnaty® (Pfizer/ BioNTech)) and V4vac-con (male, 78 years-3x Comirnaty® (Pfizer/ BioNTech)) being a couple, suffered from acute COVID-19 infections notwithstanding the booster vaccination. Disease onset despite initial immunization might be an insufficient immune resistance, i.e., an inadequate SARS-CoV-2 IgG antibody titre to protect from an acute infection, as was the case in volunteer V4vac-con, who suffered from a severe disease progression associated with a boost of the antibody titre during acute infection in comparison to V3vaccon, who showed only mild symptoms of the disease. Coefficients of Variation (CV) of vaccinated participants ranged from 2.5 to 8% over the whole study and the CV for duplicates was 0.52%. The time course of immunization for 5 volunteers is shown in Figure 3, including an unvaccinated volunteer designated as unvaccinated control. Despite various shapes of time courses, it can be observed that after the initial immunization, all volunteers dramatically increased antibody titres. However, it should be noted that in most volunteers, titres were not stable and started to decrease right after the peak. This cohort´s oldest volunteer (V5) showed the slightest increase of SARS-CoV-2 antibody titres, which decreased shortly after the third immunization below the level, promising safety to protect from COVID-19 infections. As expected, the participant who refused vaccination, i.e., the unvaccinated control, remained far below the cutoff level of 100 BAU/mL according to the assay instruction.
Table 1:Comparison of SARS-CoV-2 antibody titres of paired samples from serum and DBS of three volunteers (A [female, 49 years - 1x Vaxzevria® (AstraZeneca); 1x Spikevax® (Moderna Biotech); 1x Comirnaty® (Pfizer/BioNTech)], B [female, 32 years - 2x Vaxzevria® (AstraZeneca); convalesced], and C [female, 47 years - 1x Spikevax® (Moderna Biotech); 1x Comirnaty® (Pfizer/BioNTech)]). Mean values of two determinations are expressed as BAU/mL. Intraindividual differences between serum and DBS are presented as “Δ Percentage”. The sum of the individual deviations was reported as “Δ SUM” in percent.

Figure 1:Correlation between DBS SARS-CoV-2 IgG antibody titres and time in days after the initial immunization (r = 0,9179) from a single volunteer (male; 67 years; 2x Vaxzevria® (AstraZeneca).

Figure 2:SARS-CoV-2 antibody titres (BAU/mL) from DBS in four volunteers in a time course of 7 weeks after booster vaccination versus booster immunization plus disease. Volunteers number one (V1vac, female, 72y [3x Comirnaty® (Pfizer/BioNTech)] and number two (V2vac, male 67y [2x Vaxzevria® (AstraZeneca), 1x Comirnaty® (Pfizer/BioNTech)]) have been vaccinated three times. Volunteers number three (V3vac-con, female 59y [3x Comirnaty® (Pfizer/BioNTech)]) and number four (V4vac-con, male 78 y [3x Comirnaty® (Pfizer/BioNTech)]) were vaccinated and convalesced. V4vac-con suffered from a severe course in contrast to V3vac-con.
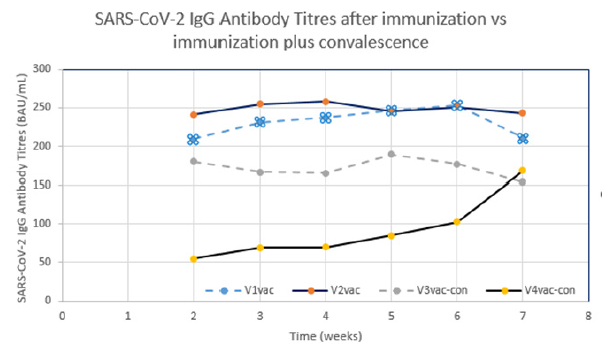
Figure 3:Follow up of SARS-CoV-2 antibody titres from DBS for several weeks in 6 volunteers, i.e., without-or after vaccination with different vaccines. Volunteer (V1) male 25 years-[unvaccinated]; V2 male 67y-[2x Vaxzevria® (AstraZeneca), 1x Comirnaty® (Pfizer/BioNTech)]; V3 male 56y-[3x Comirnaty® (Pfizer/BioNTech)]; V4 female 55y-[2x Vaxzevria® (AstraZeneca), 1x Comirnaty® (Pfizer/BioNTech)]; V5 female 89y-[3x Comirnaty® (Pfizer/BioNTech)]; V6 female 63y-[1x JCOVDEN® (Janssen), 1x Comirnaty® (Pfizer/BioNTech)].
Discussion
This study evaluated the possibility of monitoring SARS-CoV-2 antibody titres using Dry Blood Spots (DBS). The main finding of this study indicates that DBS excisions of a diameter of 3 mm gave very similar results compared with serum levels of the same person, which corresponds to the high correlation between plasma and DBS samples [18]. This underlines the robustness of DBS cards regarding the measurement of SARS-CoV-2 antibody titres and confirms the validity of this blood sampling procedure, consistent with a previous report indicating high correlations between DBS and plasma antibody titres [13]. It was also found that self-collected DBS and professionally collected DBS correlated to a great extent and met the FDA guidance with NPA (negative percent agreement of ≧95.0%) and PPA (positive percent agreement of ≧90.0%) [19]. The ELISA method was slightly modified for DBS samples, i.e., the first incubation with diluted serum samples (30 minutes at 37 °C) was substituted by elution of the blood-sodden filters (3mm in diameter) in the wells of the microtitration plates for 120 minutes at room temperature, to allow proper extractions of detectable SARS-CoV-2 IgG antibody titres. All other assay operations remained unchanged. This modification achieved comparable results in the case of duplicates and the coefficient of variation. Sample quality [20] and the choice of the assay for the analysis of SARS-CoV-2 antibodies is essential in this regard, as significant differences in sensitivity and negative predictive value have been described for commercial assays [21]. The results were in line with our hypothesis with a few exceptions, e.g., the fact that even booster vaccinations were necessary to get sufficient immunization was unexpected but was achieved with each vaccine of different manufacturers in the present study and meanwhile confirmed by others [11, 22, 23], especially for older age, immunosuppressive therapy and males. Maybe the most convincing finding was the high correlation concerning the increase of SARS-CoV-2 antibody titres after the booster vaccination. The weakest immune response was observed in the oldest female volunteer (89y), that corresponds to the interrelationship between frailty and immunity which is associated with a lower immunization [24]. Weak antibody response to an initial immunization was associated with a severe COVID-19 infection, concurrent with previous findings [17,25], in the oldest male volunteer (78y), which then boosted the antibody titre. Another indication of the validity of receiving reliable results by monitoring DBS excisions is underlined by the low SARS-CoV-2 antibody titres below the cut-off level (<100 BAU/mL) throughout the study case of the unvaccinated control, who refused vaccination and did not get sick with COVID-19. The decrease of the IgG antibody titre beyond reaching the peak corresponds to previous reports with a rapid decline between the third and sixth months [26]. The main advantage of DBS screening is the safe handling of these samples due to their minimal biohazard potential, even if they comprise originally contagious pathogens. Moreover, straightforward self-collection and transport, as well as storage at ambient temperature, designates DBS as a robust blood sampling procedure to gather the immunization state against COVID-19 in the population as a helpful information for the public health authority. SARS-CoV-2 antibodies are preserved for up to 6 months on DBS cards at room temperature, as shown previously [13]. The vulnerability of the DBS collection system is the blood application. If there is insufficient blood to soak the filter paper of the collecting unit sufficiently, it causes false negative results. Therefore, care must be taken to soak the filter papers with an adequate blood volume, i.e., between fifteen to thirty micro-liter of whole blood, easily harvested from a finger pulp. Therefore, religious groups like Mormons may be included in DBS screening programs. Post et al. [27] mentioned in their systematic review that long-term antibody dynamics are not well established, and sero-surveillance is necessary for disease control policy regarding antibody titre waning.
Conclusion
We conclude that DBS screening is a feasible alternative to conventional blood sampling systems as venipuncture for the screening of successful immunizations and the waning of antibody titres against SARS-CoV-2. DBS screening is a safe blood sampling device due to the minimal biohazard potential, straightforward self-collection and transport, and storage at ambient temperature for the surveillance of the population´s immunity, especially when people should stay away from each other. Further investigations are necessary to confirm the results of this pilot study, e.g., clinical studies with a high number of cases for the determination of DBS, serum and plasma and respective correlations.
Credit Authorship Contribution Statement
Franz Tatzber: Conceptualization, Data curation, Investigation, Project administration, Validation, Supervision, Writing-original draft, Writing-review & editing.
Ulrike Resch: Conceptualization, Methodology, Data generation, Writing-review & editing.
Matthias Kuper: Conceptualization, Methodology, Writingreview & editing.
Chittaranjan Bhaduri: Conceptualization, Methodology, Writing-review & editing.
Willibald Wonisch: Visualization, Funding, Writing-original draft, Writing-review & editing.
Gerhard Cvirn: Writing-review & editing.
Funding
The authors work was kindly supported by Fresenius-Kabi Austria with an unrestricted grant.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper, except Kuper M. who is affiliated with LDN and Bhaduri C. who is affiliated with RD-Ratio Diagnostics.
Dedication
This work is dedicated to the victims of the COVID-19 pandemic who died of the baneful effect of SARS-CoV-2 or are suffering from long-COVID.
References
- Murchu EO, Byrne P, Walsh KA, Carty PG, Connolly M, et al. (2021) Immune response following infection with SARS-CoV-2 and other coronaviruses: A rapid review. Rev Med Virol 31(2): e2162.
- Zhao J, Yuan Q, Wang H, Liu W, Liao X, et al. (2020) Antibody responses to SARS-CoV-2 in patients of novel coronavirus disease 2019. Clin Infect Dis 71(16): 2027-2034.
- Altawalah H (2021) Antibody responses to natural SARS-CoV-2 infection or after COVID-19 vaccination. Vaccines 9(8): 910.
- Worm M, Ring J, Klimek L, Jakob T, Lange L, et al. (2021) Covid-19 vaccination and risk of anaphylaxis- recommendations for practical management. MMW Fortschr Med 163(1): 48-51.
- Teymouri M, Mollazadeh S, Mortazavi H, Ghale-Noie ZN, Keyvani V, et al. (2021) Recent advances and challenges of RT-PCR tests for the diagnosis of COVID-19. Path Res Pract 221: 153443.
- Hassan F, Albahli S, Javed A, Irtaza A (2022) A robust framework for epidemic analysis, prediction and detection of COVID-19. Front Publ Health 10: 805086.
- Mim F, Reza MS, Khan MJR, Karim N, Rahman MA, et al. (2021) Evaluation of sensitivity and specificity of three commercial real-time quantitative polymerase chain reaction kits for detecting SARS-CoV-2 in Bangladesh. Cureus 13(12): e20627.
- Kadyrova I, Yegorov S, Negmetzhanov B, Kolesnikova Y, Kolesnichenko S, et al. (2022) High SARS-CoV-2 seroprevalence in KARAGANDA, KAZAKHSTAN before the launch of COVID-19 vaccination. Plos One 17(7): e0272008.
- Rnjak D, Ravlić S, Šola AM, Halassy B, Šemnički J, et al. (2021) COVID-19 convalescent plasma as long-term therapy in immunodeficient patients? Transfus Clin Biol 28(3): 264-270.
- Daniel J, Thangakunam B, Isaac BTJ, Moorthy M, Christopher DJ (2021) Recurrent COVID-19 infection in a case of rituximab-induced hypogammaglobulinaemia. Respirol Case Rep 10(1): e0891.
- Tillmann F-P, Figiel L, Ricken J, Still H, Korte C, et al. (2021) Evolution of SARS-CoV-2-neutralizing antibodies after two standard dose vaccinations, risk factors for non-response and effect of a third dose booster vaccination in non-responders on hemodialysis: A prospective multi-centre cohort study. J Clin Med 10(21): 5113.
- Markwalter CF, Nyunt MH, Han ZY, Henao R, Jain A, et al. (2021) Antibody signatures of asymptomatic Plasmodium falciparum malaria infections measured from dried blood spots. Malar J 20: 378.
- Itell HL, Weight H, Fish CS, Logue JK, Franko N, et al. (2021) SARS-CoV-2 antibody binding and neutralization in dried blood spot eluates and paired plasma. Microbiol Spectr 9(2): e01298-21.
- Comeau AM, Pitt J, Hillyer GV, Landesman S, Bremer J, et al. (1996) Early detection of human immunodeficiency virus on dried blood spot specimens: Sensitivity across serial specimens. Women and infants transmission study group. J Pediatr 129(1): 111-118.
- Wong MP, Meas MA, Adams C, Hernandez S, Green V, et al. (2022) Development and implementation of dried blood spot-based COVID-19 serological assays for epidemiologic studies. Microbiol Spectr 10(3): e0247121.
- Higgins RL, Rawlings SA, Case J, Lee FY, Chan CW, et al. (2021) Longitudinal SARS-CoV-2 antibody study using the easy check COVID-19 igm/iggtm lateral flow assay. PloS One 16(3): e0247797.
- De la Monte SM, Long C, Szczepanski N, Griffin C, Fitzgerald A (2021) Heterogenous Longitudinal antibody responses to COVID-19 mRNA vaccination. Chapin K. Clin Pathol 14: 1-9.
- Brinc D, Biondi MJ, Li D, Sun H, Capraru C, et al. (2021) Evaluation of dried blood spot testing for SARS-CoV-2 serology using a quantitative commercial assay. Viruses 13(6): 962.
- Miesse PK, Collier BB, Grant RP (2022) Monitoring of SARS-CoV-2 antibodies using dried blood spot for at-home collection. Sci Rep 12(1): 5812.
- Mulchandani R, Brown B, Brooks T, Semper A, Machin N, et al. (2021) Use of dried blood spot samples for SARS-CoV-2 antibody detection using the roche elecsys® high throughput immunoassay. J Clin Virol 136: 104739.
- Cholette F, Mesa C, Harris A, Ellis H, Cachergo K, et al. (2021) Dried blood spot specimens for SARS-CoV-2 antibody testing: A multi-site, multi-assay comparison. Plos One 16(12): e0261003.
- Levin EG, Lustig Y, Cohen C, Fluss R, Indenbaum V, et al. (2021) Waning immune humoral response to BNT162b2 COVID-19 vaccine over 6 months. N Engl J Med 385(24): e84.
- Chiarella SE, Jenkins SM, Smith CY, Prasad V, Shakuntulla F, et al. (2022) Predictors of seroconversion after coronavirus disease 2019 vaccination. Ann Allergy Asthma Immunol 129(2): 189-193.
- Chen L-K (2021) COVID-19 vaccination and frailty in older adults. Arch Gerontol Geriatr 96: 104487.
- Vogrig M, Berger A-E, Bourlet T, Waeckel L, Haccourt A, et al. (2022) Monitoring of both humoral and cellular immunities could early predict COVID-19 vaccine efficacy against the different SARS-CoV-2 variants. J Clin Immunol 1-15.
- Lemos C, Ferreira S, Gouveia C, Mendonca E, Mota AM, et al. (2021) Severe acute respiratory syndrome corona virus 2 antibodies among healthcare workers after vaccine administration in an intensive care unit. Cureus 13(12): e20579.
- Post N, Eddy D, Huntley C, Post-Eddy-Huntley-Van Schalkwyk, Shrotri M, et al. (2020) Antibody response to SARS-CoV-2 infection in humans: A systematic review. PLoS One 15(12): e0244126.
© 2022 Willibald Wonisch. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






