- Submissions

Full Text
COJ Biomedical Science & Research
Assessment of Liver Enzyme Abnormality among Antiretroviral Therapy Experienced HIVSeropositive Patients in Asmara, Eritrea
Achila OO1, Abrhaley F2, Kesete Y1,3*, Tesfaldet F4, Alazar F5, Fisshaye L6, Gebremeskel L7, Mehari R8 and Andemichael D1
1Orotta College of Medicine and Health Sciences, Eritrea
2National Health Laboratory, Eritrea
3Nakfa Hospital, Eritrea
4Afabet Hospital, Eritrea
5Ghindae Zonal Referral Hospital, Eritrea
6Halibet National Referral Hospital, Eritrea
7Keren Zonal Referral Hospital. Eritrea
8Agordot Hospital, Eritrea
*Corresponding author: Yafet Kesete, Orotta College of Medicine, Health Sciences and Nakfa Hospital, Nakfa Asmara, Eritrea
Submission: April 05, 2022; Published: May 18, 2022

Volume2 Issue2May 2022
Abstract
Background: Liver disease are predominant in HIV/AIDS patients and a wide range of the population have been affected. However, studies that have assessed the burden and risks of liver enzyme alterations among antiretroviral therapy experienced patients are hardly available in Eritrea. Thus, the present study targets to determine the prevalence and the risks related with abnormal liver enzymes among HIV-infected individuals.
Methodology: A cross-sectional, observational study was conducted in two national referral hospitals, in Asmara, Eritrea. A structured predesigned questionnaire was employed to capture sociodemographic data of patients and blood sample was taken for analyses of liver enzyme profile tests. Data was analyzed using chi-square test and logistic regressions in SPSS software.
Result: The study included 329 participants of whom majority were females. Participants’ age ranges from 18 to 83 years with the mean age of 44.63(±48). Patients had a history of taking first line regimen as either AZT, TDF, ABC or D4T based drug combinations with mean duration on drugs of 7.09(±3.32) years. 88(26.7%) HAART experienced patients had significant alterations in their liver enzyme parameters. This abnormality was significantly associated with age (p-value=0.022). HIV-1 patients with history of stavudine use as first line HAART medication had about three (AOR=2.95; 95% CI=1.19-7.27) times more elevated liver enzymes. Generally, liver enzymes were found to be higher in minority ethnic groups and rural areas (p value=0.002).
Conclusion: The results suggest that liver enzymes (ALT, AST) were significantly elevated in patients taking HAART medication. The finding of this study illustrates that HIV patients on antiretroviral medication are at increased risk of hepatotoxicity which necessitate for continuous and periodical clinical monitoring to reduce severe effects of liver injury.
Keywords: HIV; AIDS; HAART; Tenofovir; Stavudine; ALT; AST; Hepatotoxicity
Abbreviations: ART: Antiretroviral Therapy; EFV: Efavirenz; HBV: Hepatitis B Virus; HCV: Hepatitis C Virus; NNRTI: Nonnucleoside Reverse Transcriptase Inhibitor; NRTI: Nucleoside Reverse Transcriptase Inhibitor; NVP: Nevirapine; PI: Protease Inhibitor; SSA: Sub-Saharan Africa
Introduction
Liver disease are predominant in HIV/AIDS patients and a wide range of the population (25%-90%) have been reported to be affected [1,2]. More than half of deaths that occur among hospitalized HIV infected patients admitted to hospitals after global initiation of HAART medication have been associated with liver disorders [3,4]. The main causes include consequent chronic hepatitis B and C infections, opportunistic infections, AIDS related neoplasms [5,6], nonalcoholic fatty liver disease, alcohol abuse and medication-related hepatotoxicity. This leads to conditions ranging from asymptomatic moderate liver enzymes alterations to end stage liver disease and cirrhosis with its concomitant complications (i.e, hepatic encephalopathy, ascites and esophageal abnormality). An estimated overall prevalence of 8.3% of liver cirrhosis is observed in HIV population [7]. Moreover, cirrhosis incidence and liver disorder related deaths are substantially increased in hepatitis B and C infected HIV population [8-10]. Nevertheless, the burden and the risk factors of liver disorders might be distinct in various geographical locations including Eritrea. Hepatotoxicity is often demonstrated by biochemical alterations in the liver function tests. Liver enzymes (aspartate aminotransferase or alanine aminotransferase) are often elevated by several HAART drugs including NRTIs, NNRTIs and PI groups [11-13]. Half of the patients does not manifest any clinical symptoms in spite of abnormalities in their liver enzymes, therefore, in many settings cases of HIV-patients with liver disorder are underreported [14]. Liver disorder treatment and management is an integral aspect of the HIV patient’s care. Previous studies which give information on the patterns of liver function health are hardly available in Eritrea. Thus, the present study targets to determine the prevalence and the risks related with abnormal liver enzymes among HIV-infected individuals.
Methods
Study setting and design
This was a cross-sectional, observational study carried out in Halibet National Referral Hospital (HNRH) and Orotta National Medical Surgical Referral Hospital (ONMSRH), in Asmara, Eritrea from March to June, 2018. They are the largest national referral hospitals with catchment area of over 814,000 inhabitants and with simultaneously large number referral patients visiting from around the country. The study population were known HIV/AIDS positive individuals who visited ONMSRH and HNRH for routine check-up and medication. The current number of patients who were on HAART in ONMSRH and HNRH was 1567 and 1162 respectively.
Sampling technique
The study was carried out with a sampling method of convenience in the sense that, participants were patients who turned up voluntarily for their routine and subsidized semester check-up to which liver function profile tests were planned along with other laboratory monitoring tests. Study participants were HIV patients who were >18 years old and patients having good adherence to medication.
Sociodemographic data collection
A structured predesigned questionnaire was employed to gather sociodemographic information (sex, age, educational level, area of residence). Medical history of subjects (drug combination, CD4 count, viral load count, duration of exposure to HAART, and drug history and side effects) were collected from patient’s medical record with the help of hospital counselling staffs. It also incorporated questions related to the lifestyles of participants (dieting, exercise, smoking, and frequency of alcohol intake).
Specimen collection and analysis
After applying standard antiseptic technique, a total of 5ml of venous blood sample was obtained in a uniquely labelled chemistry tubes from each individual. Blood specimen were then allowed to clot and serum was separated by centrifugation at 3000 rpm for 3 minutes. The serum samples were stored at 60C and were analyzed within 24 hours of collection for Liver function tests including ALT, AST, ALP, and Bilirubin using AU480 Chemistry Analyzer (Beckman Coulter- AU480). An upper limit normal (ULN) values of 31IU/L and 35IU/L were specified for AST for women and men respectively. Similarly, upper limit normal for ALT was set as 45IU/L for men and 34IU/L for women. Abnormal liver enzyme were defined values more than 1.25 of the upper limit of normal [15].
Statistical analysis
The data were collected, cleaned and analyzed using SPSS version 20. Summary and descriptive statistics were computed. Bivariate and multivariate logistic regression analysis were employed to define the association between independent variables and the outcome variable. Moreover, Odds ratio calculations with 95%CI were used to determine the strength of association. A p-value <0.05 was set to hold statistical significance.
Ethical approval and consent to participate
Approval for the study was sought from the Asmara College of Health Science research ethical committee and Health Research Ethics and Protocol Review Committee of the Ministry of Health. Patients were given information regarding the nature and objectives of the study, then, a written and verbal consent was obtained from voluntary study participants upon the acquisition of the data and specimen.
Result
Sociodemographic characteristics. A total of 329 HIV/AIDS patients on HAART were enrolled in this study from whom majority were females and urban dwellers. The participants’ age ranges from 18 to 83 years with the mean age of 44.63(±10.48). At least half of the study subjects were literate (Table 1). All of the patients had a history of taking first line regimen as either AZT, TDF, ABC or D4T based drug combinations with mean duration on drugs of 7.09(±3.32) years. Out of 329 patients enrolled in the study, 203(61.7%) patients had changed the drug combination they use while 126 patients adhered with only one drug combination since the initiation of HAART. Prevalence of liver abnormalities. Eighty-eight (26.7%) HAART experienced patients were present with a significant alteration in their liver enzyme parameters (ALT and/or AST). AST, ALT, and both AST and ALT were increased in 84 (25.5%), 35(10.6%), and 31 (9.4%) of HAART experienced groups, respectively. Majority of the patients CD4 cell count were in the range of 200 and 500, and 45 (28.8%) of the participants in this group were present with hepatotoxicity. Factors associated with liver abnormalities. Presence of abnormal liver enzymes was significantly related with age (p-value=0.022). Those patients in the age group of 40-50 were three (AOR=3.29; 95% CI=1.29-8.39) times more likely to have increased liver enzymes in comparison with other patients (Table 2). Different types of HAART regimen were also significantly associated with presence of liver injury. Generally, stavudine(D4T) based drug regimen use was present with higher proportion of liver enzyme abnormalities. HIV-1 patients with history of stavudine use as first line HAART medication had about three (AOR=2.95; 95% CI=1.19-7.27) times more elevated liver enzyme compared to their counterparts. Moreover, though attenuated in the adjusted regression analysis, presence of lamivudine and tenofovir in drug regimen was also related with increased AST values (p value=0.04). Generally, liver enzymes were found to be higher in minority ethnic groups and rural areas (p value=0.002).
Table 1:Patients characteristics and frequency of liver injury, Eritrea 2018.
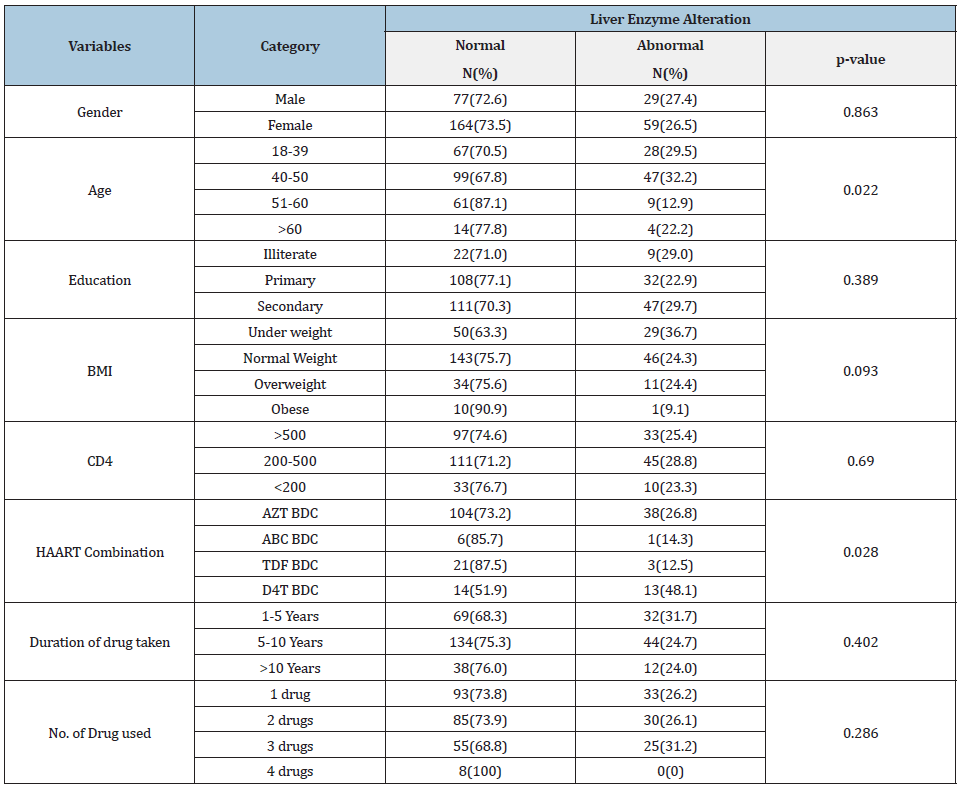
Source: AZT: Zidovudine; ABC: Abacavir; TDF: Tenofovir; BDC: Based Drug Combination; BMI: Body Mass Index.
Table 2:Multivariate logistic regression analysis of factors associated with liver injury in HIV-1 infected patients in Asmara, Eritrea 2018.
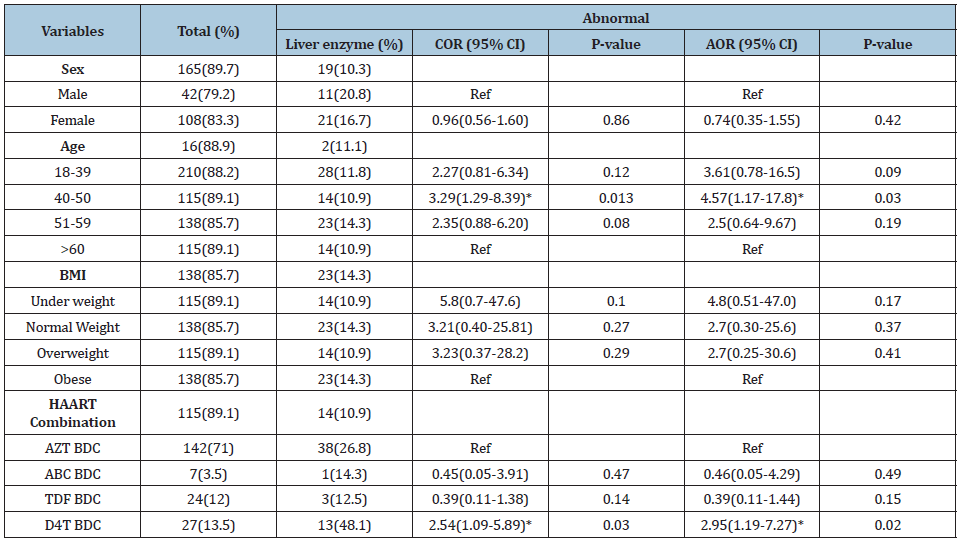
Source: *Significant at p<0.05; AZT: Zidovudine; ABC: Abacavir; TDF: Tenofovir; BDC: Based Drug Combination; BMI: Body Mass Index.
Discussion
Elevations in liver enzymes are frequent in HIV population, however, because of the complexities related to pathophysiologic mechanisms of liver function, diagnosis or general management of cases may be challenging [16]. The degree of liver cell toxicity is usually evaluated by measuring serum transaminase enzymes levels or activity [17]. It is obvious that Liver is the synthesis organ of the major body enzymes and when there is any injury on the liver, variable concentrations of those enzymes are released into the blood due to raised permeability [18]. AST and ALT are the primarily sensitive biomarkers of liver cell injury and used for the detection of hepatocellular disorders Invalid source specified. ALT is principally synthesized in liver cells, whereas AST is secreted in liver and other complementary tissues including heart, brain, lungs, kidneys, pancreas, skeletal muscles, erythrocytes and leukocytes Invalid source specified. Different research outputs indicated that patients on different HAART drugs had demonstrated raised levels of AST and AL [19-21]. In this study, the prevalence of liver injury was 26.7% in general agreement with studies in Sub-Saharan Africa (SSA) [22,23]. However, this outcome is higher to studies on HIV infected patients conducted in Cameroon (22.6%), South Africa (23%) and Brazil (19.7%) [21,24,25]. This might be attributable to different factors including traditional risks such as hepatitis B and C virus co-infections [11], increased alcohol intake [12], use of illicit regimens, the time period of treatment [26], older age, genetic predispositions and geographic conditions [27]. In concordance with other similar studies, this study indicated that liver injury was observed more in males than in females which could be due to the effect of alcohol consumption [28]. Also studies carried out in Thailand [29], US community [30]. and Italy [31]. indicated a parallel association. Analysis of other sociodemographic and clinical factors also showed a more significant association (p<0.05) between liver enzyme elevations and HAART regimen in addition to age of participants. Liver enzyme abnormalities were higher in the age group 40 to 50 years. This is comparable to several findings elsewhere [32,33]. Different HAART regimen have been directly implicated in alterations of liver enzyme activity [11,34]. In this study, zidovudine and tenofovir were used by large portion (83%) of study participants as first line regimen for their HAART medication. But a high proportion of the liver enzyme abnormality (13.3%) was present in HIV patients who had history of stavudine based medication (or=2.95; 95% CI=1.19-7.27). This is consistently supported by previous animal study and clinical trials conducted demonstrating induction of some extent of long-term mitochondrial toxicity [35,36]. WHO guidelines strictly urge the termination of wide use of stavudine in first-line regimens for reason related to its well documented metabolic toxicities. A complete phasing out of stavudine as preferred drug regimen has been obtained in Eritrea recently. However, still less than 5% of the HIV population on antiretroviral treatment uses this drug worldwide [37].
Limitations
In this study, several limitations can be mentioned. Regardless of very low national prevalence of HCV and HBV, hepatitis virus coinfection along with other opportunistic diseases were not evaluated in this study. A typical liver function parameter such as albumin activity and INR was not included which could have enabled for an elaborate analysis in the organ mechanisms. Ultrasound analysis and platelet count were not assessed for the detection of liver fibrosis and ascites. It is hugely understood that those tests are expensive and for very low-income countries like Eritrea, it is impractical to implement all the tests.
Conclusion
In this study, liver enzymes (ALT,AST) were markedly raised in patients on antiretroviral medication. Moreover, hepatotoxicity was noticed in a considerable number in older HIV participants and on those who had history of stavudine medication, imparting an inevitable issue to be addressed. The results of this study point out that HIV patients on HAART medication are at eminent risk of liver toxicity which necessitate for continuous clinical monitoring to reduce complicated form of liver injury.
References
- Wnuk AM (2001) Liver damage in HIV-infected patients. Medical Science Monitor 7(4): 729-236.
- Dieterich DT, Robinson PA, Love J, Stern JO (2004) Drug induced liver injury associated with the use of nonnucleoside reverse-transcriptase inhibitors. Clinical Infectious Diseases 38(2): 80-89.
- Bica I, McGovern B, Dhar R (2001) Increasing mortality due to end-stage liver disease in patients with human immunodeficiency virus infection. Clinical Infectious Diseases 32(3): 492-497.
- Mart-Carbonero L, Soriano V, Valencia E, Samaniego JG, Lopez M, et al. (2001) Increasing impact of chronic viral hepatitis on hospital admissions and mortality among HIV-infected patients. AIDS Research and Human Retroviruses 17(16): 1467-1471.
- Cappell MS (1991) Hepatobiliary manifestations of the acquired immune deficiency syndrome. The American Journal of Gastroenterology 86(1): 1-15.
- Lefkowitch JH (1994) Pathology of AIDS-related liver disease. Digestive Diseases 12(6): 321-330.
- Castellares C, Barreiro P, Carbonero LM (2008) Liver cirrhosis in HIV-infected patients: Prevalence, aetiology and clinical outcome. Journal of Viral Hepatitis 15(3): 165-172.
- Bodsworth N, Donovan B, Nightingale BN (1989) The effect of concurrent human immunodeficiency virus infection on chronic hepatitis B: A study of 150 homosexual men. Journal of Infectious Diseases 160(4): 577-582.
- Gilson RJC, Hawkins AE, Beecham MR (1997) Interactions between HIV and hepatitis B virus in homosexual men: Effects on the natural history of infection. AIDS 11(5): 597-606.
- Walsh EK (1999) Clinical outcomes after hepatitis C infection from contaminated anti-D immune globulin. Irish hepatology research group. The New England Journal of Medicine 340(16): 1228-1233.
- Sulkowski MS, Thomas DL, Mehta SH, Chaisson RE, Moore RD (2002) Hepatotoxicity associated with nevirapine or efavirenz-containing antiretroviral therapy: Role of hepatitis C and B infections. Hepatology 35(1): 182-189.
- Kovari H, Ledergerber B, Battegay M (2010) Incidence and risk factors for chronic elevation of alanine aminotransferase levels in HIV-infected persons without hepatitis B or C virus co-infection. Clinical Infectious Diseases 50(4): 502-511.
- Wit FW, Weverling GJ, Weel J, Jurriaans S, Lange JMA (2002) Incidence of and risk factors for severe hepatotoxicity associated with antiretroviral combination therapy. Journal of Infectious Diseases 186(1): 23-31.
- Aranzabal L, Casado JL, Moya J (2005) Influence of liver fibrosis on highly active antiretroviral therapy-associated hepatotoxicity in patients with HIV and hepatitis C virus coinfection. Clinical Infectious Diseases 40(4): 588-593.
- (2017) US Department of Health and Human Services, Division of AIDS (DAIDS) Table for Grading the Severity of Adult and Pediatric Adverse Events, Division of AIDS, National Institute of Allergy and Infectious Diseases, National Institutes of Health, US Department of Health and Human Services.
- Pol S, Lebray P, Pichard AV (2004) HIV infection and hepatic enzyme abnormalities: Intricacies of the pathogenic mechanisms. Clin Infect Dis 38(2): 65-72.
- Carlo T, Giuseppe L, Salvatore C, Massimo P, Mark N, et al. (2005) Incidence and risk factors for liver enzyme elevation during highly active antiretroviral therapy in HIV-HCV co-infected patients; results from the Italian EPOKA-Master Cohort. BMC Inf Dis 5: 58.
- Burtis C, Ashwood E, Bruns D, Tietz (2006) Textbook of clinical chemistry and molecular diagnosis, Philadelphia: Elsevier Saunders Co, US.
- Marc G, Jay H, Fauci S, Braunwald E, Kasper L, et al. (2008) Harrison’s principles of internal medicine. 17th (edn.), McGraw-Hill Companies, USA, pp. 1918-2000.
- Hernandez L, Gilson I, Jacobson J, Affi A, Puetz T, et al. (2001) Antiretroviral hepatotoxicity in human immunodefciency virus-infected patients. Aliment Pharmacol Ther 15(10): 1627-1632.
- Hofmann C, Charalambous S, Thio C, Martin D, Pemb L, et al. (2007) Hepatotoxicity in an African antiretroviral therapy cohort: The effect of tuberculosis and hepatitis B. AIDS 21(10): 1301-1308.
- Tesfa E, Siefu D, Belayneh Y, Mekonnen Z (2019) Liver enzyme elevation in patients taking HAART compared with treatment naïve controls at Debre Berhan Referral Hospital: A comparative cross-sectional study, Northeast Ethiopia. BMC research notes 12(1) :714.
- Shiferaw MB, Tulu KT, Zegeye AM, Wubante AA (2016) Liver enzymes abnormalities among highly active antiretroviral therapy experienced and HAART naive HIV-1 infected patients at debre tabor hospital, north west Ethiopia: A comparative cross-sectional study. AIDS Research and Treatment 2016: 1-7.
- Lucent K, Clement A, Fon N, Weldeji P, Ndikvu C (2010) The effects of antiretroviral treatment on liver function enzymes among HIV infected out patients attending the central hospital of Yaounde Cameron. African Journal of Clinical and Experimental Microbiology 11(3): 174-178.
- Gil ACM, Lorenzetti R, Mendes GB (2007) Hepatotoxicity in HIV-infected children and adolescents on antiretroviral therapy. Sao Paulo Medical Journal 125(4): 205-209.
- Hussaini S, Farrington E (2007) Idiosyncratic drug-induced liver injury; An overview. Expert Opin Drug Saf 6(6): 673-684.
- Yimer G, Amogne W, Habtewold A, Makonnen E, Ueda N, et al. (2012) High plasma efavirenz level and CYP2B6*6 are associated with efavirenz-based HAART-induced liver injury in the treatment of naive HIV patients from Ethiopia; A prospective cohort study. Pharmacogenomics J 12(6): 499-506.
- Ocama P, Castelnuovo B, Kamya M (2010) Low frequency of liver enzyme elevation in HIV-infected patients attending a large urban treatment centre in Uganda. Int J STD AIDS 21(8): 553-557.
- Chalermchai T, Hiransuthikul N, Tangkijvanich P, Pinyakorn S, Avihingsanon A, et al. (2013) Risk factors of chronic hepatitis in antiretroviral-treated HIV infection, without hepatitis B or C viral infection. AIDS Research and Therapy 10(1): 1-9.
- Kim WR, Benson JT, Therneau TM, Torgerson HA, Yawn BP, et al, (2004) Changing epidemiology of hepatitis B in a US community. Hepatology 39(3): 811-816.
- Guaraldi G, Squillace N, Stentarelli C (2008) Nonalcoholic fatty liver disease in HIV-infected patients referred to a metabolic clinic: Prevalence, characteristics, and predictors. Clinical Infectious Diseases 47(2): 250-257.
- Pokorska ŚM, Stańska-Perka A, Popielska J, Ołdakowska A, Coupland U (2017) Prevalence and predictors of liver disease in HIV-infected children and adolescents. Scientific Reports 7: 1-8.
- Pathania MS, Kaur SLN, Kumar MS, Sashindran ACV, Puri BP (2017) A cross-sectional study of liver function tests in HIV-infected persons in Western India. Medical Journal Armed Forces India 73(1): 23-28.
- Rose A, Abraham O, Bode J, Abel N (2011) Risk factors for hepatotoxicity after introduction of highly active antiretroviral therapy. E&C Hepatol 7(1): 49-56.
- Ahmed M (2006) Abacavir induced reversible fanconi syndrome with nephrogenic diabetes insipidus in patients with acquired immunodeficiency syndrome. J Postgrad Med 52(4): 296-297.
- Krishnan M, Nair R, Haas M, Atta M (2000) Acute renal failure in HIV positive 50-year-old man. Am J Kidney Dis 36(5): 1072-1078.
- (2016)WHO “Clinical guidelines: Antiretroviral therapy” in Consolidated guidelines on the use of antiretroviral drugs for treating and preventing HIV infection, Geneva: World Health Organization.
© 2022 Yafet Kesete. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






