- Submissions

Full Text
COJ Biomedical Science & Research
Marine Sponges (Scypha): Potential Source for Secondary Metabolites
Vibha Bhardwaj*
Director Environment Laboratories, RAK Municipality, UAE
*Corresponding author:Vibha Bhardwaj,Director Environment Laboratories, RAK Municipality, Ras Al Khaimah, UAE
Submission: February 01, 2022; Published: March 28, 2022

Volume2 Issue1March , 2022
Abstract
Infectious diseases are responsible for millions of global deaths annually. The misuse and increasing failure of chemotherapy and antibiotic resistance exhibited by bacterial pathogens has prompted researchers for screening of new antimicrobials from prolific producers of novel natural products including marine sponges [Scypha]. In the present study, crude extracts of marine sponge in methanol was investigated for secondary metabolites (flavonoids, glycosides, phenols, saponins, tannins, terpenoids) and antibacterial effect of marine sponge was evaluated on (multidrug resistant MDR) strains of Bacillus subtilis (ATCC 6633), E. coli (ATCC 8739), Salmonella enterica (ATCC 14028), Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 27853) by agar well diffusion method. Ciprofloxacin was used as standard.
The methanolic extracts of Scypha tested positive for glycosides and terpenoids. Maximum zone of inhibition was observed with Bacillus subtilis which is 23±0mm and Staphylococcus aureus which is 20±0mm. The potency shown by these extracts recommends their use against multidrug resistant microorganisms. The present study suggests that the methanol extract of the marine sponge (Scypha) exhibited a potential antibacterial activity against the tested microorganisms and could be a potential source of new antimicrobial agents as well as secondary metabolites.
Keywords:Marine sponges; Antibacterial; Scypha; Multidrug resistant; Microorganism
Keywords:SD: Standard Deviation; MDR: Multidrug Resistant; ATCC: American Type Culture Collection; E: Extract 1; H: hours; C: Ciprofloxacin
Introduction
More than 70% of the surface of earth covered by Oceans and is still to a great extent unexplored. The expansion of new technologies that facilitate sub-sea sampling and harvesting has widened the accessibility to areas below sea level Synnes [1]. Marine flora and fauna produce many biologically active molecules that have applications in pharmaceuticals, cosmetics, molecular probes, enzymes, nutritional supplements, fine chemicals, and agrochemicals. Several members of marine microorganisms produced important secondary metabolites, including antibiotics, herbicides, and growth-promoting substances Bertrand et al. [2]. Overuse of antimicrobial agents has been attributed to the development of multidrugresistant (MDR) microorganisms, which increases the mortality rate associated with infectious diseases Singer et al. [3] and Magiorakos et al. [4]. Efforts to find new effective antimicrobials from alternative sources is because of multidrug resistance and associated mortality. Scientists have continuously searched for isolation of novel bioactive compounds from marine organisms against viral, bacterial, parasitic and fungal diseases (Abdel mohsen et al. [5], Andersen [6] and Anjum et al. [7] and also to overcome drug resistance’s inherent problems, the toxicity of currently used compounds, and the increased incidence of severe diseases, which have been reported to cause cancer, antimicrobial resistance, and neurodegenerative pathologies which have led to the need for the discovery and development of novel medicines.
Sponges are the most primitive multicellular animals and are spineless belonging to the phylum Porifera. Marine sponges are soft-bodied, sessile, and filter feeders by assembling small particulate matter from seawater rising through their bodies (Figure 1) Kamaruding et al. [8]. All over the world, Marine sponges are gaining more attention by researchers and industrial sectors due to their ability of production of bioactive secondary metabolites which have many applications Garcia-Vilas et al. [9], Kobayashi [10] and Mioso et al. [11].
Figure 1:Marine sponge.

It was discovered that novel antimicrobials are developed from different nano compounds Wang et al. [12] and Makvandi et al. [13]. However, approximately 50% of the new drugs originated from natural products; it was decided to explore marine microorganisms Webster et al. [14]. The production of any bioactive and mass cultivation of marine sponges being difficult, due to the requirement for large amounts of sponge biomass, so the utilization of sponges as a source of antibiotics is hindered Indraningrat et al. [15]. The observed structural similarities among active compounds from marine sponges and terrestrial microorganisms indicated that sponge associated bacteria could be the main sources of some of these active compounds Taylor et al. [16]. Moreover, numerous studies have identified a wide range of antimicrobial activities from sponge-associated microbes, which makes these microbial populations an important source for novel antimicrobials Graca et al. [17].
Many efforts have been made to discover new antimicrobial compounds from various kinds of sources such as plants, animals and microorganisms Khan et al. [18], Gibbons [19] and Gottlieb [20]. Natural products, either as pure compounds or as standardized extracts, provide unlimited opportunities for new drug leads because of the unmatched availability of chemical diversity. The need for new, safer, and effective therapeutic agents represents the main targets for clinical investigators Kujawska et al. [21]. There is a lack of data on the antibacterial and antifungal activity of marine invertebrates’ compounds because, marine invertebrates have been mostly researched for neurophysiological and anticancer properties rather than antimicrobial potential. Therefore, most of the research done on the isolation of marine-associated microbiota for antimicrobial compound screening. United Arab Emirates’ has rich biodiversity of marine invertebrates across its far-stretching coastline, this was a perfect opportunity for marine natural products to be explored from marine sponge species indigenous to the Coastline of Ras Al Khaimah, UAE.
In our previous study we investigated about the nutraceutical, antimicrobial properties of ghaf Al Ghais et al. [22-24] and Bhardwaj V [25,26]. Also, we investigated that ghaf and mangrove has potential of antioxidant and antimicrobial properties Bhardwaj V [27-29]. Therefore, to continue our further research to detect the potency of marine natural source for new antimicrobial agent and also to meet the increasing demand of antimicrobial agent, alternative strategies, this study have been considered recently. Therefore, Hence, the objectives of the study were to study the antimicrobial activity of methanolic extract of sponge and also secondary metabolites. This research was carried out as an awareness of medicinal value of marine sponges.
Material and Methods
Sponge collection
The sponge Scypha was collected in month of October 2021, from coastline of the Arabian Sea at Ras Al Khaimah, United Arab Emirates. The freshly collected sponge were cleaned, washed and taken to the laboratory in seawater and immediately frozen at -20 °C until use. Weight of sponge, before preservation was 122.4gms.
Preparation of the extracts
The frozen samples were left to defrost for one hour at room temperature. Weight of sponge, after preservation was 138.6gms. Crude extracts were extracted following the method of Braekman et al. [30] with some modifications. Defrosted samples (20g) were then dissected using scissors into small pieces, 200mL of methanol as well as deionized water were added and kept standing for 48 hours. Solvents were then removed, by squeezing sponge samples, and filtered through Whatman filter paper number 1. Transferred the extract for drying for 30min and finally got residue of sponge sample. Therefore, 100mL of methanol was added to dried residue, mixed well. The resultant compound was transferred into small vials and labeled and stored at 4 °C in a refrigerator for further analysis as crude methanolic extracts.
Chemicals
The chemicals used in the present investigation were of analytical grade and of high purity from Merck. Standard kits and reagents used for analysis were purchased from Germany and USA.
Test organisms
In the present study, the bacterial strains used were Bacillus subtilis (ATCC 6633), E. coli (ATCC 8739), Salmonella enterica (ATCC 14028), Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 27853) obtained from the American Type Culture Collection (ATCC) to determine the antibacterial activity of Scypha. The bacterial strains were procured from LTA srl Italia. Pure culture of bacteria was maintained at 4 °C on nutrient agar slants.
Methodology for detection of antibacterial activity
Inoculums preparation: The bacterial isolates were first grown in 5ml of nutrient broth into sterile test tubes for 18h before use.
Agar well diffusion assay:The antibacterial activity of methanolic extracts of Scypha was tested against isolates by agarwell diffusion method. An aliquot of 100μl inoculum for each bacterial isolate was evenly spread by a sterile glass spreader onto Muller Hinton Agar using sterilized cotton swab and was allowed at room temperature. A Cork borer of 6mm diameter was used to punch well in agar plates to cut uniform wells. Wells were bored in agar plates. The concentration of the extract was 10% (w/v), prepared using methanol as solvent. Subsequently, 30μl extracts of bark were poured into the wells. Ciprofloxacin 30μg was used as positive control. Then the plates were kept at 2-8 °C in a refrigerator to allow diffusion of the extracts into the agar and further incubated at 37 °C for 24h. The diameter of zone of inhibition was measured to the nearest millimeter Sohel [31] and Uddin et al. [32]. The formation of clear inhibition zone of ≥7mm diameters around the wells was regarded as significant susceptibility of the organisms to the extract Okwori [33]. The effect was compared to those of antibiotic discs. The tests were performed in triplicates and the mean was taken. The whole experiments were performed under strict aseptic conditions.
Phytochemical analysis
Test for Flavonoids (Ammonia test):1ml of the extract was taken in the test tube and ammonia solution was added (1:5) followed by the addition of conc. sulphuric acid. Appearance of yellow color and its disappearance on standing indicates the positive test for flavonoids
Test for Glycosides (Keller Kiliani test):5ml of each extract was added with 2ml of glacial acetic acid which was followed by the addition of few drops of ferric chloride solution and 1ml of conc. sulphuric acid. Formation of brown ring at interface confirms the presence of glycosides.
Test for Phenols (Ferric chloride test):0.5ml of the extract was added with few drops of neutral ferric chloride (0.5%) solution. Formation of dark green color indicates the presence of the phenolic compounds.
Test for Saponins (Froth test):1ml of the extract was taken in a test tube and distilled water (2ml) was added to it. The test tube was then kept in boiling water bath for boiling and was shaken vigorously. Existence of froth formation during warming confirms the presence of saponins.
Test for Tannins (Ferric chloride test):1ml of the extract was added with 5ml of distilled water and kept for boiling in hot water bath. After boiling, sample was cooled down and to this 0.1% ferric chloride solution was added. Appearance of brownish green or blue-black coloration confirms the presence of tannins.
Test for Terpenoids (Salkowski test):5ml of extract was taken in a test tube and 2ml of chloroform was added to it followed by the addition of 3ml of conc. sulfuric acid. Formation of reddishbrown layer at the junction of two solutions confirms the presence of terpenoids.
Statistical analysis
The tests were performed in triplicates. Data are expressed as mean. Pair wise comparisons were performed. Experimental error was determined for triplicate and expressed as standard deviation (SD).
Result
Antibacterial activity of sponges extracts against Human pathogenic bacteria
Table 1 summarizes the results of antibacterial activities of extracts of marine sponge, which was evaluated on multidrug resistant (MDR) strains of Bacillus subtilis (ATCC 6633), E. coli (ATCC 8739), Salmonella enterica (ATCC 14028), Staphylococcus aureus (ATCC 6538), Pseudomonas aeruginosa (ATCC 27853) by agar well diffusion method. Strong activity was detected by Bacillus subtilis which is 23±0mm whereas Staphylococcus aureus exhibited moderate-good activity, which was detected 20±0mm (Figure 2). All tested extracts of marine sponges (Scypha) showed no activity against E. coli, Salmonella.enterica, Pseudomonas aeruginosa (Table 1).
Table 1: Diameters of the inhibition zone to extracts of Scypha.
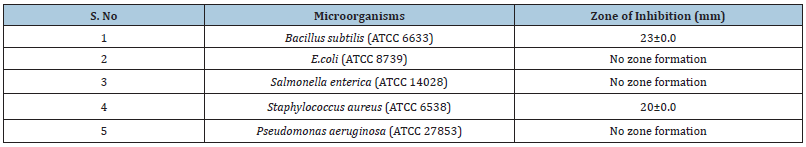
Figure 2: Extracts of marine sponge Scypha showed antibacterial activity as indicated by the zone of inhibition against different microorganism’s strain.
Phytochemical screening of marine sponge species
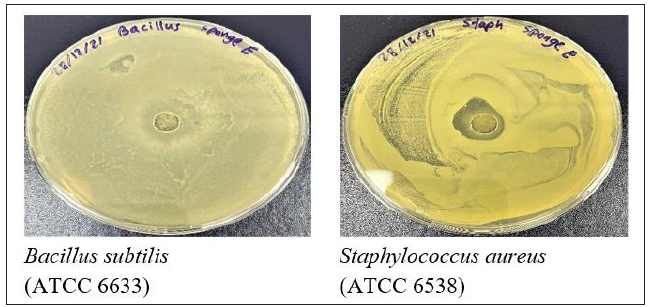
Six phytochemicals were screened for this research work (tannins, phenolic, terpenoids, glycosides, saponins and flavonoids) as seen in Table 2, from the crude extracts obtained from marine species exhibiting bioactivity. Scypha methanol crude extract only tested positive for the presence of terpenoids and glycosides. Scypha methanol crude extract did not test positive for the existence of any of the other mentioned phytochemicals in Table 2 and Figure 3.
Figure 3:1 and 2 showing the confirmation of terpenoids and glycosides present in crude extract of Scypha.
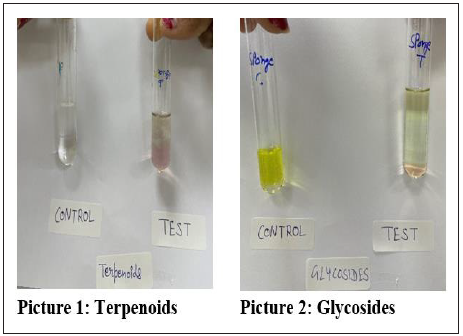
Table 2: Phytochemicals present in bioactive methanolic crude extracts of Marine sponge (Scypha).

Discussion
The objective of this research was to screen crude extracts of sponges derived from Ras Al Khaimah coastline, for their activity against selected bacterial pathogens. Similar results were revealed by Antelmann et al. [34] and Chang et al. [35] which showed that extracts derived from Callyspongia crassa and Aplysina fulva were found potent against tested Gram-positive human pathogens. These data are of importance, especially with the increasing threat of bacterial antibiotic resistant in both hospital settings and the food industry. The discovery of useful antimicrobial compounds is gaining ground. There is necessitates for alternative therapeutic agents for disease management (World Health Organization, 2020) because antimicrobial resistance is an existing problem. Marine invertebrates have been proven in some parts of the world as good sources of antifungal, antibacterial, larvicidal, anticancer, and anti-inflammatory compounds Alves et al. [36] and Cita et al. [37] reported that methanolic crude extracts of Clathria sp. collected from Pasir Putih, East Java (Indonesia), were not bioactive against P. aeruginosa and MRSA. In our study, similar results were obtained. Said et al. [38] reported that the species of Tedania did not show bioactivity against C. albicans, S. aureus, and P. aeruginosa. Our study recorded, contrasting result of bioactivity against the S. aureus
Marine sponges are known to produce diverse groups of phytochemicals, including alkaloids, diterpenoids, sesquiterpenoids, and steroids Wei et al. [39]. These different bioactive compounds have unique biological functions and have been identified to possess promising therapeutic applications Mushtaq et al. [40]. Our study evaluated the phytochemicals present in the crude extracts of marine species Scypha against human pathogenic bacteria. Similar to our study, Weerasinghe et al. [41] reported the absence of flavonoids in their Clathria sp. Samples; In contrast to our study, he reported the presence of alkaloids and saponins in their ethanolic crude extracts. Most studies have evaluated the presence of phytochemicals in plants, with less work being done regarding evaluating the potential antimicrobial properties of sponges found in oceans [42].
Conclusion
The broad-spectrum antibacterial activity of marine sponges seemed to be due to the presence of terpenes, glycosides detected in the bioactive fractions. This work confirms the hypothesis on the chemical richness of sponges and is the research data on the antimicrobial activity of Scypha from Ras Al Khaimah coastline, to the best of our knowledge. These promissory extracts open the possibility of finding new clinically effective antibacterial compounds as well as secondary metabolites.
Availability of Data and Materials
The relevant data and materials are available in the present study.
Acknowledgement
Authors would like to thank all individuals who provided their efforts for this research especially Aaesha Ahmed Alzaabi, for her assistance during research work.
References
- Synnes M (2007) Bioprospecting of organisms from the deep sea: scientific and environmental aspects. Clean Technol Environ Policy 9(1): 53-59.
- Bertrand B, Munoz-Garay C (2019) Marine antimicrobial peptides: A promising source of new generation antibiotics and other bio-active molecules. International Journal of Peptide Research and Therapeutics 25: 1441-1450.
- Singer RS, Finch R, Wegener HC, Bywater R, Walters J, et al. (2003) Antibiotic resistance-The interplay between antibiotic use in animals and human beings. Lancet Infect Dis 3(1): 47-51.
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, et al. (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: An international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3): 268-281.
- Abdelmohsen UR, Balasubramanian S, Oelschlaeger AT, Grkovic T, Pham NB, et al. (2017) Potential of marine natural products against drug-resistant fungal, viral, and parasitic infections. Lancet Infect Dis 17(2): 30-41.
- Andersen RJ (2017) Sponging off nature for new drug leads. Biochem Pharmacol 139: 3-14.
- Anjum K, Abbas SQ, Shah SA, Akhter N, Batool S, et al. (2016) Marine sponges as a drug treasure. Biomol Ther (Seoul) 24(4): 347-362.
- Kamaruding NA, Ismail N, Sokry N (2020) Identification of antibacterial activity with bioactive compounds from selected marine sponges. Pharmacognosy Journal 12(3): 493-502.
- Garcia-Vilas JA, Martinez-Poved B, Quesada AR, Medina MA (2015) Aeroplysinin-1, a sponge-derived multi-targeted bioactive marine drug. Mar Drugs 14(1): 1.
- Kobayashi J (2016) Search for new bioactive marine natural products and application to drug development. Chem Pharm Bull (Tokyo) 64(8): 1079-1083.
- Mioso R, Marante FJ, Bezerra RS, Borges FV, Santos BV, et al. (2017) Cytotoxic compounds derived from marine sponges. A review (2010-2012). Molecules 28(22): 208.
- Wang C, Makvandi P, Zare EN, Tay FR (2020). Advances in antimicrobial organic and inorganic nanocompounds in biomedicine. Adv Ther 3(8): 2000024.
- Makvandi P, Wang C, Zare EN, Borzacchiello A, Niu, L, et al. (2020) Metal-based nanomaterials in biomedical applications: antimicrobial activity and cytotoxicity aspects. Adv Funct Mater 30(22): 1910021.
- Webster NS, Taylor MW (2012) Marine sponges and their microbial symbionts: Love and other relationships. Environ Microbiol 14(2): 335-346.
- Indraningrat AAG, Smidt H, Sipkema D (2016) Bioprospecting sponge-Associated microbes for antimicrobial compounds. Mar Drugs 14(5): 87.
- Taylor MW, Radax R, Steger D, Wagner M (2007) Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev 71(2): 295-347.
- Graça AP, Viana F, Bondoso J, Correia MI, Gomes L, et al. (2015) The antimicrobial activity of heterotrophic bacteria isolated from the marine sponge Erylus deficiens (Astrophorida, Geodiidae). Front Microbiol 6: 389.
- Khan R, Islam B, Akram M, Shakil S, Ahmed A, et al. (2009) Antimicrobial activity of five herbal extracts against multi drug resistant (MDR) strains of bacteria and fungus of clinical origin. Molecules 14(2): 586-597.
- Gibbons S (2005) Plants as a source of bacterial resistance modulators and anti-infective agents. Phytochem Rev 4: 63-78.
- Gottlieb OR, Borin MR, Brito NR (2002) Integration of ethnobotany and phytochemistry: dream or reality? Phytochemistry 60(2): 145-52.
- Kujawska M, Pardo-De-Santayana (2015) Management of medicinally useful plants by European migrants in South America. Journal of Ethnopharmacology 172 :347-355.
- Ghais S, Bhardwaj V, Kumbhar P (2020) Prosopis cineraria (ghaf): An unconventional desert protein rich supplement. American Journal of Agricultural Research 5: 94.
- Ghais S, Bhardwaj V, Kumbhar P (2020) Prosopis cineraria (ghaf): A potential desert nutraceutical. International Journal of Development Research 10(3): 34162-34165.
- Ghais Saif, Bhardwaj V, Kumbhar Pramod (2020) Antimicrobial properties of prosopis cineraria stem bark. American Journal of Microbiology and Immunology 5(7).
- Bhardwaj V (2021) Unprecedented antimicrobial properties of prosopis cineraria International Journal of Engineering Applied Sciences and Technology 6(1): 285-288.
- Bhardwaj V (2021) Pods of prosopis cineraria (ghaf): a gift of nature for nutraceutical. Journal of Global Ecology and Environment 11(1): 15-18.
- Bhardwaj V (2021) Inherent antioxidant potential of avicennia marina leaves. International Research Journal of Modernization in Engineering Technology and Science 3(6).
- Bhardwaj V (2021) Avicennia Marina: A novel convivial phyto medicine for antibiotic resistant pathogenic bacteria. J Biomed Stud 1(101).
- Bhardwaj V (2021) Antioxidant properties of prosopis cineraria (ghaf): Pods and Leaves. International Journal of Scientific Research & Engineering Trends 7(3).
- Braekman JC, Daloze D, Stoller C, Van Soest RWM (1992) Chemotaxonomy of Agelas (Porifera: Demospongiae). Biochem Syst Ecol 20(5): 417-431.
- Sohel A (2010) Antibacterial activity of the ethanol extracts of hibiscus rosa-sinensis leaves and flowers against clinical isolates of bacteria. Bangladesh J Life Sci 22(2): 65-73.
- Uddin B, Nahar T, Khalil MI, Hossain S (2007) In vitro antibacterial activity of the ethanol extracts of Paederia Foetida L. (rubiaceae) leaves. Bangladesh J life Sci 19(2): 141-143.
- Okwori AE (2007) Antibacterial activities of Ageratum conyzoides extracts on selected bacterial pathogens. Internet J Microbiol 4(1): 34-56.
- Antelmann H, Hecker M, Zuber P (2008) Proteomic signatures uncover thiol-specific electrophile resistance mechanisms in Bacillus subtilis. Expert Rev Proteomics 5(1): 77-90.
- Chang VS, Dhaliwal DK, Raju L, Kowalski RP (2015) Antibiotic resistance in the treatment of Staphylococcus aureus Keratitis: a 20-year review. Cornea 34(6): 698-703.
- Alves E, Dias M, Lopes D, Almeida A, Domingues M, et al. (2020) Antimicrobial lipids from plants and marine organisms: an overview of the current state-of-the-art and future prospects. Antibiotics (Basel) 9(8): 441.
- Cita YP, Muzaki FK, Radjasa OK, Sudarmono P (2017) Screening of antimicrobial activity of sponges extracts from Pasir Putih, East Java (Indonesia). Journal of Marine Science 7(237): 2
- Said SA, Moshi MJ, Nondo RSO, Masimba PJ, Innocent E, et al. (2010) Evaluation of the potential of the marine sponges of the Zanzibar Island to yield antimalarial and antimicrobial active compounds. Tanzania Journal of Health Research 12(3): 195-20.
- Wei X, Nieves K, Rodríguez A D (2010) Neopetrosiamine a, biologically active bis-piperidine alkaloid from the caribbean sea sponge neopetrosia proxima. Bioorganic & Medicinal Chemistry Letters 20(19): 5905-5908.
- Mushtaq S, Abbasi BH, Uzair B, Abbasi R (2018) Natural products as reservoirs of novel therapeutic agents. EXCLI Journal 17: 420-451.
- Weerasinghe RL, Ranatunga RR, Chinthaka DM, Marasinghe MM (2019) Toxicity evaluation and volatile component analysis of tropical marine sponge Clathria sp. (Schmidt, 1862). International Journal of Marine Sciences 6(11): 17-25.
- World Health Organization (2020).
© 2022 Vibha Bhardwaj. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






