- Submissions

Full Text
COJ Biomedical Science & Research
The Effect of Benzyladenine and Indolebutyric Acid on Shooting and Rooting of Miniature Rose
Gholami M1, Khorshidi M1* and Agrawal V2*
1School of Biology, Damghan University, Damghan, 3671641167, Iran
2Associate Professor, International Research Center of Spectroscopy and Quantum Chemistry- IRC SQC, Siberian Federal University, Krasnoyarsk, 660041, Russia
*Corresponding author: Khorshidi M, School of Biology, Damghan University, Damghan, 3671641167, Iran, E-mail: m_ khorshidi@du.ac.ir Agarwal V, Associate Professor, International Research Center of Spectroscopy and Quantum Chemistry-IRC SQC, Siberian Federal University, Krasnoyarsk, Russia 660041, E-mail: dr.vikashpro@yahoo.com
Submission: June 02, 2021; Published: June 29, 2021

Volume2 Issue1June 2021
Abstract
In vitro propagation is one of the essential propagation methods and growth of rose (Rosa miniature). In this study, we used young, healthy shoots harboring one or two axillary buds of miniature rose as explants and MS medium as a basal medium containing several concentrations (0,0.5,1,2, and 4mg/L) of 6-Benzyl adenine (BA) and Indole-3-butyric acid (IBA) as growth regulators on the shoot and root regeneration. Our results illustrated that the highest number and most extended shoots were observed on MS medium containing 1mg/L BA. In MS medium with 0 and 0.5mg/L BA, the main shoot just could be grown without any multiplicities. BA had a positive effect on the enhancement of shoot induction. For rooting, a maximum number of rooted shoots were obtained on MS medium supplemented with 1mg/L IBA. Our observation showed the indispensable role of IBA during the rooting stage. In conclusion, we have established a tissue culture system by which micro propagated rosa miniature was rooted in vitro successfully
Keywords: Benzyladenine; Indolebutyric acid; Micropropagation; Miniature rose
Introduction
Miniature roses belong to the Rosaceae family, and the genus rosa is trendy because of its stunning beauty and has many commercial uses [1]. In different parts of the world, a large number of hybrids and cultivars of genus rosa are widely used as garden plants, cut flowers, potted plants and for the perfume industry [2,3]. Roses propagated by vegetative methods like budding, cutting, layering, grafting and in some cases by seed [4,5] which are challenging, undesirable and time-consuming [3].
Although these conventional methods are a dominant way in rose, they don’t warrant healthy and disease-free plant and also, environmental events cause the cultivar to degenerate gently [6]. They also caught by frequently cutting from mother plants subjected to infection and in addition to that increasing production costs [7]. Micropropagation through in vitro culture has been developed as an applicable and satisfactory method for rapid and mass propagation in several plant species and many crops’ cultivars [8].
Tissue culture is assumed an asexual propagation technique since it only includes the cells from a single parent plant and generates genetically equal plants to the parent plant and to each other [9]. Cytokinins and auxins are two crucial hormones that used as plant growth regulators [10]. These hormones cause to boost the division and specialization of cells and the development of stems, leaves and roots [11,12].
Rout et al. [13] stated that the presence of cytokinin in the culture medium helped the multiplication of shoots in hybrid roses year-round. In most of the reports, varying concentrations of different auxins used for root induction [14] and prevent the cutting death [15]. Our main objective of this study was to investigate the effect of five different concentrations of two hormones and to peruse the number and length of stems and roots as well as the number of leaves. In this work, axillary buds were utilized as explant in MS medium on the shoot and root proliferation in rose miniature.
Material and Methods
The shoots of rosa miniature were collected from the flower production and breeding area of Pakdasht, Varamin and investigated in the tissue culture laboratory of the faculty of plant biology, Damghan University. At first, the shoots were cut, and all leaves and injured branches were separated. Thereafter, they were washed with running water to remove the superficial dust followed by detergent water with constant shaking for 3min and surface sterilized by dipping into 70% ethanol for 1min and incubated in 10% sodium hypochlorite (5.25% NaOCl) solution of commercial laundry bleach containing 2 drops of Tween-20 emulsifier to aid wetting for 20 minutes. Finally, they were rinsed with sterile distilled water for 3 to 5 times.
The shoots were cut in 5-6cm length harboring 1or 2 axillary buds. Full strength MS medium [16] containing macro and microelements, salts, vitamins, 3% sucrose, 0.7% agar was used in this study. The pH of the culture was adjusted to 5.7 using NaOH or HCl before autoclaving. The medium was distributed into a culture jar (50cc per jar) and autoclaved at 121.5c at 1.5atm for 20 minutes. The shoots containing 1 or 2 buds explanted on MS medium in jars under culture room condition at 23 ± 2c under white fluorescent light with 16 hours photoperiod. The explants were subculture on the same media every week. Shoots were grown after four weeks. In MS medium without any growth regulators, the shoots can grow 1 to 3cm in length without any multiplicities. The young, healthy shoots were excised and cultured on the experimental mediums for shoot regeneration in a laminar flow cabinet. The experimental medium for shoot regeneration consisted of MS medium supplemented with 0, 0.5, 1, 2 and 4mg/L BA. The culture medium for rooting stage containing MS medium with 0, 0.5, 1, 2 and 4mg/L IBA.
Statistical Analysis
The number of shoots and roots were evaluated after each culture cycle was 4 weeks. Three explants were placed per culture and 25 cultures were raised for each treatment. All experiments were carried out in three replicates. Morphological data were taken after four weeks and analyzed by analyses of variance. Significant differences were determined at the (P<0.05) were assessed using Duncan’s multiple range test.
Results and Discussion
In the shooting experiment, the effects of different concentration of BA on shoot multiplication and elongation are presented in Table 1.
Table 1: Effects of BA concentrations (mg/L) on shoot regeneration, length of shoots, no of shoots/explant, no of leaves of rosa miniature. Values represent the means ± S.E. means followed by the same different letters are not significantly different at p<0.01.
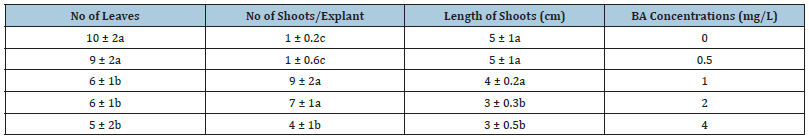
Figure 1:In vitro culture of rosa miniature. a) Growth in the main shoot without any multiplicities on MS medium with 0mg/L BA. b) Highest number of regenerated shoots on MS medium with 1mg/L BA. c) Lowest shoots on MS medium with 0.5mg/L BA. d) Regeneration of shoots on MS medium with 4mg/L BA.

The length of shoots reduced with increasing BA concentration. There was no significant difference in shoots length per explant; However, higher levels of BA (2 or 4mg/L) were less effective in shoot elongation compared to 0mg/L. The tallest shoots were observed on MS medium without BA. In contrast, the effects of increasing BA concentration were different meaningfully on shoots number in all of the treatments. The highest number of shoots observed on MS medium containing 1 and 2mg/L BA and the lowest was on MS medium with 0.5mg/L BA. In MS medium without BA, the main stem could be grown but shoot multiplication wasn’t considered (Figure 1).
Kim et al. [17] showed that cytokinin is significant plant growth regulators in invitro shoot proliferation and multiplication of roses. Although several different cytokinins have been used in rose proliferation but the best proliferation rate was obtained using BA [18-20]. BA has been used to experiment on shoot multiplication of several rose species [21]. In this experiment, the highest number of leaves achieved on MS medium culture with 0mg/L BA. The leaves number decreased with increasing BA concentrations per explant and half of the leaves are green and the other half are yellow.
To establish root proliferation, single green and regenerated shoots were separated and transferred to MS medium enriched with different concentrations of IBA (3 explants per culture). IBA has been suggested as the best plant growth regulator to induce the rooting of rose explants [22]. The effects of different concentration of IBA on regeneration and length of root showed in Table 2. The maximum number of roots and roots length were obtained from MS medium supplemented with 1 and 2mg/L IBA, while the minimum number of roots and roots length were observed on medium with 4mg/L IBA.
Table 2: Effects of IBA concentrations (mg/L) on root regeneration, length of the stem, no of leaves, no of roots/explant, length of roots of rosa miniature. Values represent the means ± S.E. means followed by the same different letters are not significantly different at p<0.01.
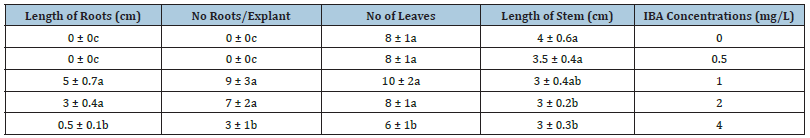
These results agreed with Asad et al. [23] that showed MS medium with 1mg/L IBA proved best root induction in rose. Root regeneration and elongation weren’t observed on MS medium with 0 and 0.5mg/L IBA (Figure 2). There are considerable differences in root number and length when using IBA as a growth regulator. Arnold et al. [24] reported a relatively similar effect of IBA on root formation of rose in vitro. IBA is also the most effective and widely used for rooting plants and these results supported by Ozel et al. [25] investigation. Also, it was observed that the number of the rooted shoot and leaves reduced with increasing in IBA concentration and this reduction is noticeable on MS medium with 4mg/L IBA.
Figure 2:The effect of different concentrations of IBA on rooting of rosa miniature. a) 1mg/L IBA b) 2mg/L IBA c) 0mg/L IBA d) 0.5mg/L IBA e) 4mg/L IBA.

Conclusion
In conclusion, this study provided an in vitro propagation protocol for rosa miniature. The results reveal that the maximum number of shoots was achieved in the medium containing 1mg/L BA. At the same time, optimum root regeneration occurs in MS medium supplemented with 1mg/L IBA, whereas lower or higher concentrations inhibited it. Micro-propagated plants were rooted and established in vitro successfully. This protocol will help for mass propagation, horticulture and aromatic or pharmaceutical industries.
References
- Austin D (2005) Botanica roses (The encyclopedia of roses). Random House Australia.
- Short KC, Roberts AV (1991) Rosa (roses): In vitro culture, micropropagation and the production of secondary products. In: Bajaj YPS (Ed.) Biotechnology in agriculture and forestry. Medicinal and aromatic plants III. Springer, Berlin, Germany, 15: 376-397.
- Horn WAH (1992) Micropropagation of roe (Rosa L). In: Bajaj YPS (Ed.), High-tech and micropropagation IV. Springer, Berlin, Germany, 20: 320-342.
- Saleh H, Khui KM (1997) Effects of explant length diameter on in vitro shoot growth and proliferation rate of miniature roses. Journal Horticultural Science 72: 673-676.
- Azadi P, Beyramizadeh E, Ntui OV (2013) A simple protocol for somatic embryogenesis in Rosa hybrid cv. Apollo. The journal of Horticultural Science and Biotechnology 88(4): 399-402.
- Roy PK, Mamun ANK, Ahmed G (2004) In vitro plantlets regeneration of rose. Plant Tissue Culture 14(2): 149-154.
- Hahn EJ, Boe JH, Lee YB (1998) Growth and leaf surface characteristics of chrysanthemum plantlets in micropropagation and microponic system. Journal of Korean Society Horticulture Science 39: 838-842.
- Khan IA, Shaw JJ (1998) Biotechnology in agriculture. Punjab Agric Res Coordination Board. Faisalabad, Pakistan.
- Kane M (1991) Rose flowers: The tissue culture approach. American Rose Magazine.
- Fishel FM (2009) Plant growth regulators.
- Hyndman SE, Hasegawa PM, Bressan RA (1982) Stimulation of root initiation from cultured rose shoots through the use of reduced concentrations of mineral salts. Hort Sci 17(1): 82-83.
- Nizamani F, Nizamani G, Nizamani M, Ahmed S, Ahmed N (2016) Propagation of rose (Rosa Hybrida L.) under tissue culture technique. International Journal of Biology Research 1: 23-27.
- Rout GR, Samantary S, Mottely J, Das P (1999) Biotechnology of the rose: A review of recent progress. Sci Hort 81(3): 201-228.
- Pati PK, Sharma M, Ahuja PS (2005) Micro propagation, protoplast culture and its implications in the improvement of scented rose. Acta Hortic 547: 147-158.
- Kasim NE, Rayya A, Shaheen MA, Yehia TA, Ali EL (2009) Effects of different collection times and some treatments on rooting and chemical international constituents of bitter almond hard wood cutting. Journal of Agriculture and Biological Sciences 5(2): 116-122.
- Murashige T, Skoog F (1962) A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiologia Plantarum 15: 473-497.
- Chkou KJO, Jee SO, Chung JD (2003) In vitro Micropropagation of Rosa hybrid J Plant Biotechnol 5: 115-119.
- Attia O, Attia EL, Dessoky S, Adel E (2012) In vitro propagation of Rosa hybrida L.cv. Al-Taif rose plant. African Journal of Biotechnology 11(48): 10888-10893.
- Khui KM, Jabbarzadeh Z (2007) Effects of several variables on in vitro culture of Damask Rose (Rosa damascene Mill). Acta Horticulture 751: 389-393.
- Kumar A, Sood A, Plani UT, Gupta AK, Plani L (2001) Micropropagation of Rosa damascene Mill. From mature bashes using thidiazuron. J Horticult Sci 76: 30-34.
- Wang GY, Yuan MF, Hong Y (2002) In vitro flower induction in roses. In vitro Cellular and Developmental Biology-Plant 38(5): 513-518.
- Pati PK, Kaur N, Sharma M, Ahuja PS (2010) In Vitro propagation of ornamental plants. Jain SM, Ochatt SJ (Eds.), Humana Press, New York, USA, pp. 163-176.
- Asad S, Hameed N, Ali A, Bajwa R, Vecherko NA, et al. (2010) Factors affecting the growth and development of roses in vitro. Biotechnol Theo Prac 1: 41-52.
- Arnold NP, Michael RB, Daniel CC, Nayana NB, Raymond P (1995) Auxins, salt concentrations and their interactions during in vitro rooting of winter hardy and hybrid tea roses. Hort Sci 30(7): 1436-1440.
- Ozel CA, Arsalan O (2006) Efficient micropropagation of English shrub rose "Heritage" under in vitro International Journal of Agriculture and Biology 5: 626-629.
© 2021 Agrawal V. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






