- Submissions

Full Text
COJ Biomedical Science & Research
Human Brucellosis Among Pyrexia of Unknown Origin Patients (Both Occupationally Exposed and Unexposed) in Mymensingh, Bangladesh
Rasheduzzaman1, Hossain MA2, Paul SK1, Nasreen SA3, Haque N1, Akter A4, Tooha MK5, and Ahmed S*1
1Department of Microbiology, Bangladesh
2Department Microbiology and Mycology, Bangladesh
3Department of Microbiology, Bangladesh
4Registrar Gynae and Obstetrics, Bangladesh
5Laboratory Science & Service Division (LSSD), Bangladesh
*Corresponding author: Ahmed S, Department of Microbiology, Bangladesh
Submission: August 26, 2020; Published: December 22, 2020

Volume1 Issue4December 2020
Abstract
Background: Human Brucellosis is a major bacterial zoonotic disease worldwide. More than 500,000 new cases are reported globally every year with the annual incidence rates varying widely from <2 to >500 per1000000 population among different region of the world. The laboratory diagnosis of Human Brucellosis is based on microbiological, serological or molecular methods like PCR and real time PCR.
Objective: To determine the prevalence of Human Brucellosis among the suspected cases of Pyrexia of unknown origin (PUO).
Methods: This cross-sectional descriptive study was carried out in the department of microbiology, Mymensingh medical college, Mymensingh, Bangladesh from 1st January to 31 December 2017, on total 600 patients of PUO with or without occupational exposure. Blood was taken as sample, serum was separated and screening test was done by Brucella specific latex agglutination test from all collected samples. Titer of ≥1:160 were taken as positive. ICT, PCR and real time PCR were done from all screening positive samples.
Results: Among the study population 65.83% (395/600) were male and 34.17% (205/600) were female. The sero prevalence of Human Brucellosis was found 13.33% (40/300) and 5% (15/300), respectively in risk and non-risk group of population. Out of 55 Brucella specific latex agglutination test positive samples 27.27% (15/300) were ICT positive, 5.45% (3/55) were PCR positive and 3.64% (2/55) were real time PCR positive. Among the ICT positive cases 86.67% (13/15) from risk group and 13.33% (2/15) from non-risk group of study population. Among the 3 PCR positive cases 66.67% (2/3) were ICT test positive and 33.33% (1/3) was ICT negative. All PCR and Real time PCR positive cases were found in risk group of study population.
Conclusion: No reliable data for Human Brucellosis is available in our country. However, the present study revealed that a considerable number of Human Brucellosis is present in risk group as well as non-risk group of population in both rural and urban area in Mymensingh district of Bangladesh. In our country we use only Brucella specific latex agglutination test for diagnosis of human Brucellosis. It is a non-specific test and gives many false positive results. So, it is necessary to introduce newer tests with higher sensitivity and specificity which will help to diagnose the disease rapidly and more accurately.
Keywords: PCR; ICT; PUO; Real time PCR
Introduction
Brucellosis is a zoonotic and occupational disease reported in farmers, veterinarians, slaughterhouse workers, animal handlers and meat inspectors [1]. It is caused by bacteria belonging to genus Brucella. They are small Gram-negative, non-spore forming, non-capsulated Coccobacilli. Brucellosis is a very old zoonotic disease and recent evidence from Egyptian ancient skeletons has shown that this disease has been existing since at least 750 BC [2]. The responsible micro-organism was isolated by David Bruce in 1887 from the spleen of a soldier who died from the disease [3] since then new species have been identified. At present there are six known species of Brucellae including B. melitensis, B. abortus, B. sius, B. canis, B. ovis, B. neotomae, [4]. The disease remains endemic in many regions of the world including Latin America, the Middle East. Africa, Asia, and the Mediterranean basin International travelers visiting Brucella endemic region are at risk of infection (136). There are sporadic reports of prevalence of Human Brucellosis in Bangladesh and the true incidence of Brucellosis is not known. Serological tests done among cattle handlers of some villages in southwest part of Bangladesh showed 13% positive cases [5].
The laboratory diagnosis of Human Brucellosis is based on microbiological, serological, or molecular methods, each having its own advantages and disadvantages. Many serological tests such as Rose Bengal plate test (RBPT), complement fixation test (CFT) and serum agglutination test (SAT), Coombs test, ELISA are used for the diagnosis of Human Brucellosis but isolation of pathogen from blood culture remains the gold standard [6,7]. The molecular diagnosis of Human Brucellosis can be performed using genus specific polymerase chain reaction (PCR), molecular assay targeting the bcsp31 gene coding for a 31kDa immunogenic outer membrane protein conserved among all Brucella spp are the most common molecular targets in clinical applications [8]. Real time PCR provides a mean of detecting and quantifying DNA targets by monitoring PCR product.
Materials and Methods
This Cross-sectional descriptive type of study was carried out in the department of microbiology, Mymensingh medical college Mymensingh, Bangladesh, on patients suffering from pyrexia of unknown origin who were attending at outpatient and inpatient department of Mymensingh medical college hospital from 01/01/2017 to 31/12/2017. Total 600 (six hundred) patients of either sex (300 from risk group/occupationally exposed and 300 from non-risk group) were included in this study. 10ml Whole blood was collected aseptically from each patient from that 5ml were stored in -20 °C for PCR and Real time PCR and Serum were separated from rest of blood and used for Brucella specific latex agglutination test and ICT.
At first screening test (Brucella specific latex agglutination test) were done with all collected samples and Titer ≥1:160 are considering as screening positive [9]. The screening positive samples were then tested for ICT, PCR and real time PCR. The Brucella specific latex agglutination test (Spinreact SA/SAU. ctra Santa calona. 7E.17176 SA NT ESTEVE 1E BAS (G1) Spain) was performed on each sample according to the manufacturer’s instructions. Brucella IgM/IgG LFA is an immune chromatographic lateral flow assay (one diagnostics, 1090HA, Amsterdam, The Netherlands). The assay was intended to be used as an aid in the sero diagnosis of Brucellosis. The Brucella IgM/IgG LFA consists of two devices, one for the detection of specific IgM antibodies and one for the detection of specific IgG antibodies and the test were done according to the manufacturer’s instructions.
Polymerase chain reaction (PCR) detection of Brucella genome specific gene encoding outer membrane protein by PCR from blood was performed by using standard protocol with specific primer. Above steps were repeat for 35 cycles in automated DNA thermal cycler (Dl in, china). Final extension was done at 72 °C for 5 minutes. The PCR products were run in 1.5% agarose gels stained with ethidium bromide and visualized under the UV trans-illuminator.
Primer used for PCR
The molecular diagnosis of Human Brucellosis can be performed using genus specific primers by polymerase chain reaction (PCR) [6], molecular assay targeting the bcsp31 gene coding for a 31-kDa immunogenic outer membrane protein conserved among all Brucella spp are the most common molecular targets in clinical applications [5].
Visualization and documentation
The amplified products were visualized by electrophoresis in 1.5% agarose gel then viewed in UV light and photographs was taken by using digital camera.
Interpretation
Samples were score as positive when a PCR product of 223bp in case of 31KDa antigen genes could be detected in PCR.
Real time PCR
To detect Brucella spp by real time PCR from samples we used amplification kit manufactured by GEMEKAM Biotechnology, AG, and Germany.
Result analysis
After the end of program, the graphics were seen. In negative control it was running along with the bottom and for positive control it gave a curve in the software graphics. Real time PCR machine software used for analysis of the result (Figure 1).
Figure 31:PCR product of 31 KDa outer membrane gene.

Discussion
Human Brucellosis is a major bacterial zoonosis reported worldwide especially in developing countries. Millions of people are at risk of Brucellosis because in developing countries Infection of animals has not been under control (180). In practice blood culture is positive in 10%-30% of Human Brucellosis cases and remainder is diagnosed serologically and by molecular methods [6]. Although no single test provides 100% sensitivity and specificity, a variety of serological tests and molecular tests have been applied for diagnosis of Human Brucellosis. In the present study majority of the cases 49.17% (295/600) were in the age group between 20-40 years and total 65.83% (395/600) were male and 34.17% (205/600) were female. Male female ratio 2:1 (Table 1). The predominance of study population in age group (20-40yrs) and male was due to people in this age group are more active and the main earning member of the family in our society. So, they have more chance to occupational exposure. The sero prevalence of Brucellosis by Brucella specific latex agglutination test in risk group was 13.33 % (40/300) and non-risk group 55 (15/300) respectively (Table 2). In some other study (2,160) showed that the sero prevalence of Brucellosis were 13% and 14.8% respectively which correlated with our current study. The increased prevalence rates in risk group of population may be due to occupational contact with domestic animal and lack of personal hygiene. The prevalence of brucellosis by using IgM/ IgG (ICT) kit was 27.27% (15/55) (Table 3). All the positive cases were found positive by IgM kit. No IgG kit were found positive. This indicates the cases were acute or recent infection. In a study at Borana and from Hamar, Ethiopia [10] showed that 34.1% and 29.4% patients were sero positive for Brucellosis using Brucella immunoglobin M (IgM), lateral flow assay, which was almost similar to our current study. It appears to me no data available regarding the use of ICT IgM/IgG device for detection of Human Brucellosis in Bangladesh. In our present study we detected Brucella genome specific gene encoding outer membrane protein (LPS) (Table 4). Out of 55 sero positive samples 5.45% (3/55) were PCR positive. All positive cases were found in risk group. No cases from non-risk group were found Positive by PCR. In The present study also detected the Brucella genus species DNA by real time PCR method, the findings of real time PCR from the sero positive cases were showed in (Table 5). Out of 55 samples 3.64% (2/55) were found positive. The cut off value of real time PCR test were 0.849195 indicating a positive reaction in both situations. Out of 3 PCR positive cases 2 were real time PCR positive. The low prevalence of PCR and Real time PCR positive cases in our country may be due to that Brucellosis is not endemic in our country.
Table 1:Showing the total distribution of study population regarding age and sex. Among the study population 65.83% (395/600) were male and 34.17% (205/600) were female. Majority of the study population 49.17% (295/600) were in the age group of 20-40 years.

Table 2:Showing the result of Brucella specific latex agglutination test in both group of study population. In risk group 12% (36/300) and 1.33% (4/300) having a titer of 1:160 and 1:360 respectively, the cumulative percentage were 13.33% and in non-risk group 5% (15/300) population has a titer of 1:160.
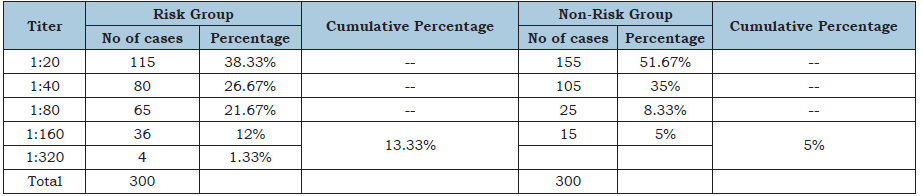
Table 3:Shows the distribution of ICT positive cases among the Brucella specific latex agglutination test positive cases. Total 15(27.27%) cases were ICT positive. Among them 13 (32.50%) were from risk group and 2 (13.33%) from non-risk group of study population.

Table 4:Shows the distribution of PCR positive cases among the Brucella specific latex agglutination test positive cases. Total 5.45% (3/55) cases were found PCR positive, all PCR positive cases were found in risk group and no cases were found positive in non-risk group of study population.
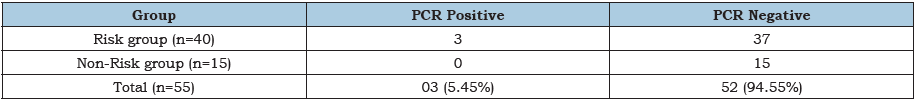
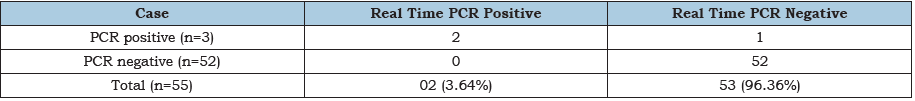
Table 5:Showing the distribution of Real time PCR positive cases among the PCR positive and PCR negative cases. Out of 55 cases 3.64% (2/55) were Real time PCR positive. No Real time PCR positive cases were found from PCR negative cases. All PCR and Real time PCR positive cases were found in risk group of study population.
Conclusion and Recommendations
The present study revealed that a considerable number of human brucellosis is present in risk group as well as non-risk group of population in both rural and urban area in Mymensingh district. More sensitive and specific test is required to diagnose the disease more rapidly and accurately, as latex agglutination test is nonspecific and gives many false positive results. Consequently, human brucellosis should be included as a differential diagnosis in PUO cases especially in PUO with history of occupational exposure. Further study on the subject from different region of Bangladesh should be carried out in long scale to find out the true prevalence of the disease.
Limitations
Non availability as well as high cost of the reagent in local market
limited the number of cases in this study.
Time constrain, the study was done in a limited period of time.
References
- Pathak AD, Dubal ZB, Doijad S, Raorane A, Rodrigues S, et al. (2014) Human brucellosis among pyrexia of unknown origin cases and occupationally exposed individuals in Goa Region, India. Emerg Health Threats J 7: 23846.
- Kazemi S (2020) A study of brucella infection in humans available online.
- Kadri SM, Rukhsana A, Laharwal MA, Tanvir M (2020) Seroprevalence of brucellosis in Kashmir (India) among patients with pyrexia of unknown origin. J Indian Med Assoc 98(4): 170-171.
- Jorgensen JH, Pfaller MA, Carro KC, Funke G, Landry ML, et al. (2015) Manual of clinical microbiology. American Society of Microbiology.
- Asaad AM, Alqahtani JM (2012) Serological and molecular diagnosis of human brucellosis in Najran, Southwestern Saudi Arabia. J Infect Public Health 5(2):189-194.
- Corbel MJ (1997) Brucellosis: An overview. Emerg Infect Dis 3(2): 213-221.
- Mufinda FC, Boinas F, Nunes C (2017) Prevalence and factors associated with human brucellosis in livestock professionals. Rev Saude Publica 51: 57.
- Navarro E, Escribano J, Fernández J, Solera J (2002) Comparison of three different PCR methods for detection of Brucella spp in human blood samples. FEMS Immunol Med Microbiol 34(2): 147-151.
- Navarro E, Casao MA, Solera J (2004) Diagnosis of human brucellosis using PCR. Expert Review of Molecular Diagnostics 4(1): 115-123.
- Lulu AR, Araj GF, Khateeb MI, Mustafa MY, Yusuf AR, et al. (1988) Human brucellosis in Kuwait: A prospective study of 400 cases. QJM An Int J Med 66(249): 39-54.
© 2020 Ahmed S. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






