- Submissions

Full Text
COJ Biomedical Science & Research
The Influence of Different Molecular Weights of Polyethylene Glycol (Peg) to the Formation of the Aqueous Two-Phase System Peg-Natrium Salt of Citric Acid-Water
Mugaddas SG*
Department of Physics, Russia
*Corresponding author: Mugaddas SG, Department of Physics, Russia
Submission: September 22, 2020; Published: November 17, 2020

Volume1 Issue3November 2020
Abstract
In presented work physico-chemical properties of PEG-C6O7H5Na3-H2O two-phase systems were studied. Building the binodal curve of this system and define the tendency angle of connecting line. Given the binodal curve of Mn=1500,3000,6000 of PEG with natrium citric acid aqueous solution the two-phase system in Decart coordinates, and connecting line, at the same time also given the equation of connecting line which defined by the method of least squares. With the influence of molecular weight of PEG to the system, obtained the binodal curve is slipped to beginning of coordinate at the low concentrations, two-phase systems are occurred with low concentration of components which formed phases. The results show that, with increasing of molecular weight of polyethylene glycol the binodal curve is splinted to beginning of coordinate, in other words, the separation of phases process occur at low concentration of polymers which formed phases.
Keywords: Aqueous two-phase systems; Binodal curve; Natrium salt of citric acid; Polyethylene glycol; Tendency angel
Introduction
When two particular chemically different polymers (e.g., dextran (Dex) and polyethylene glycol (PEG)) or one polymer and a specific salt (e.g., PEG and sodium phosphate) are mixed at certain concentrations in an aqueous solution, the solution separates into two immiscible phases. One phase is rich in one polymer, and the second phase is rich in the other polymer as (or salt) with water as a solvent in both phases [1,2].
One of the main characteristics of polymer-water two-phase systems is its binodal curve. In the below Figure 1 is the schematic phase diagram (binodal curve) of the aqueous mixture of PEG and salt (or another polymer).
Figure 1: Schematic phase diagram of ATPS. A=two phases, B=one liquid phase, C=critical point, EF=tie line, E=composition of the top phase, F=compositions of the bottom phase, and D=total composition.
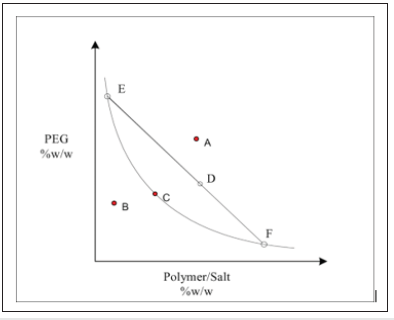
Generally, binodal of two-phase aqueous polymer systems is understand that the geometrical place of transition points from one-phase oblast to two-phase oblast in coordinate system which consist of concentration of polymers. In the Figure 1, bottom oblast from binodal curve is called one-phase or homogen oblast and top oblast is called two-phase or heterogen oblast. In other words, if we prepare the system which corresponding to any point that taken from heterogen oblast (A), this system is separated to two-phase in thermodynamic equilibrium. One of the phases of the system is enriched with anyone (for example, first phase PEG, second phase salt) of polymers.
The binodal curve and tendency angle of connecting (or tie) line are taken as a main characteristic of polymer-polymer-water two-phase systems. The connecting line (EF) in phase diagram is passes point which shows the composition of polymers of initial system that taken heterogen oblast and passes points which show that the composition of phases are corresponding to this point, existed at the same time. At the same time, polymer composition of phases which of two-phase system is the same with any point which taken on the connecting line. These phases are differing from each other for only volumes. As the result of the research works, for given pair of polymers and solvent if taken some initial system and forming contacting lines, these lines are parallel to each other.
As note that, controversial ideas can be found in the literature about the separation mechanism of phases in polymer-polymer-water and polymer-inorganic electrolyte two-phase systems. Some writers were suggested that phase formation components play main role in formation of two-phase systems. So that, if two polymer pair or polymer-salt pair are made two-phase system at any solvents, then this process must observe in other solvents. In recent years, Masimov EA [3,4], Zaslavsky B [1] and others were suggested hypothesis that the formation of two-phase aqueous polymer systems, water has a key role in scientific investigation and they were confirmed with experimental facts. They are suggest the hypothesis that phase formation components which include water, each of them influence to the structure and states of the water, and created two different structural or state water, and the same different structural waters are formed two-phase system at thermodynamic equilibrium which larger value than certain value of concentration are collected separated phases. Note that, the phases of system exist at the same time, can be accepted phase from thermodynamic. For confirmation of this hypothesis reviewed that the external influences to formation phase process (such as temperature, molecular weight of polymers, some additions and etc.).
It is possible that ionic compounds in water other substances can be dissolved in water which keep many polar groups. Dissolved in water of these matters are explained with polar groups form hydrogen bonding with water molecules.
Increasing to the dimensions of the molecules of carbohydrogens, noble gases and other non-polar compounds, they are difficulty dissolved in water and even it is impossible. The dimensions of biological and synthetic macromolecules are greater several order than dimensions of these molecules. From this, for these molecules dissolved in water they must have not ion hydrophile or have ion groups. In this case, the influence of these groups with water change to the free energy that, dissolved is possible. From this reason, the polymers which are dissolved in water have either ion groups or hydrophile nature. In practice, we can see that all polymers dissolves in water to retain 2 group: to participate group in hydrophob hydratization and created the hydrogen bonding with water or dipole-dipole influence as keeping molecules. For example, HO(-CH2-CH2)nH in PEG (-CH2-CH2) hydrophob, part and have oxygen atom (-O-) which can form the hydrogen bonding with water molecules in each core of the polymer ring. So that, as usual local structure of water around in the given macromolecule either from hydrophile part of macromolecule with water molecules that forms hydrogen bonding or created from water molecules which around non-polar part with each other relatively strong hydrogen bonding. The result of interaction of this two-effect account hydrophob-hydrophile balance of macromolecule, around this certain structure is formed. Note that, water structure around polymer molecule can’t explain with experimental physical-chemical methods [5-7]. But this local structure is enough large because this hydrate lay, we can see as microphase has characteristic structure which different from clean water structure.
So that, conclusion these above, we can come to a conclusion that, any high-molecular or low-molecular matters include water, are change the available structure and corresponding state. Beside inorganic salts, the organic salts also greater influence on water and be of great interest. One of these organic salts-C6O7H5Na3–sodium tartrate is taken as an object of research of this thesis.
According to the aqueous mixture of C6O7H5Na3 salt with PEG, to obtain two-phase system at certain concentration of components. As in the investigated two-phase aqueous polymer systems, at the same time existed phases of PEG-C6O7H5Na3-H2O systems they are keep both two components, they have different concentration in phases (see binodal curve). At this time, the interaction between water and components play main role. In this thesis physico-chemical properties of PEG-C6O7H5Na3-H2O two-phase systems were studied. For this purpose, using PEG (6000, 3000,1500), natrium salt of citric acid (chemical clean) and double-distillation water. Building the binodal curve of this system and define the tendency angle of connecting line.
In Figure 2 given the binodal curve of Mn=1500,3000,6000 olan PEG with natrium citric acid aqueous solution the two-phase system in Decart coordinates, and connecting line, at the same time also given the equation of connecting line which defined by the method of least squares.
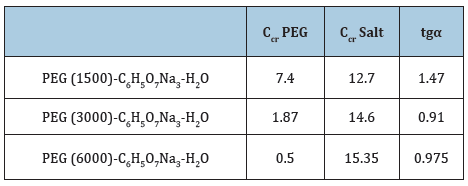
With the influence of molecular weight of PEG to the system, obtained the binodal curve is slipped to beginning of coordinate at the low concentrations, two-phase systems are occurred with low concentration of components which formed phases. The results show that, with increasing of molecular weight of polyethylene glycol the binodal curve is splinted to beginning of coordinate, in other words, the separation of phases process occur at low concentration of polymers which formed phases. In Table 1 is given the critical point compositions of aqueous two-phase system PEG (1500,3000,6000)- C6O7H5Na3-H2O.
Figure 2:
Binodal curve of PEG-C6O7H5Na3-H2O two-phase system in Decart coordinates and tie lines.
- PEG (6000)- C6O7H5Na3-H2O
- PEG (3000)-C6O7H5Na3-H2O
- PEG (1500)-C6O7H5Na3-H2

Table 1: Critical point compositions of three ATPS made with PEG and C6O7H5Na3 of different molecular weights of PEG.
As is known, investigation of two-phase aqueous polymer system is actual that is why the processes which these systems are can be accepted as the model of the processes which in living organisms. Really, the explore of biological substances separation between the phases which exist at the same time and differ from each other for hydrophobicity can help to explain metabolism mechanism that carried out with blood.
Conclusion
The formation of two-phase system is observed in aqueous mixture of polyethylene glycol with sodium salt of citric acid at thermodynamic equilibrium. Phase diagram of acquired new two-phase system (binodal curve and tendency angle of connecting or tie line) is investigated.
For clarify to the mechanism of phase separation in the PEG - sodium salt of citric acid -water two-phase system, studied the effect of molecular weight of polyethylene glycol to phase diagram. Know that, the influence of molecular weight of polyethylene glycol to water are different since the influence of them to phase diagrams of studied system is also different. It is confirmed that information of two-phase system water has main role. The results show that, with the increasing of molecular weight of PEG the binodal curve is splinted to beginning of coordinate, in other words, the separation of phases process occur at low concentration of polymers which formed phases.
References
- Zaslavsky B (1994) Aqueous two-phase partitioning: Physical chemistry and bioanalytical applications. Book review 367: 98-98.
- Albertson PA (1986) Partition of cell particles and macromolecules. (3rd edn), Wiley Online Library, New Jersey, USA, p. 396.
- Masimov EA, Bagirov TO (2016) Multicomponent multiphase systems. Laman Publishing Polygraphy, pp. 280.
- Masimov EA (2010) Physical chemistry of polymers. Baku University Publishing House, Baku, Azerbaijan, pp. 416.
- Chaplin M. Water structure and science.
- Iqbal M, Tao Y, Xie S, Zhu Y, Chen D, et al. (2016) Aqueous two-phase system (ATPS): An overview and advances in its applications. Biological Procedures Online 18.
- Masimov EA (2007) Water and living organism.
© 2020 Mugaddas SG. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






