- Submissions

Full Text
COJ Biomedical Science & Research
Investigation the Existence of H2A Gene in Leishmania Tropica
Alhabash M and Maarouf M*
Department of Biochemistry and Microbiology, Faculty of Pharmacy, Syria
*Corresponding author: Maarouf M, Department of Biochemistry and Microbiology Faculty of Pharmacy, Damascus, Syria
Submission: March 13, 2020; Published: July 20, 2020

Volume1 Issue2July 2020
Abstract
Cutaneous Leishmaniasis caused by Leishmania tropica is one of the most important health issues in Syria which exacerbated after the war in Syria. Reduction of the high prevalence of Leishmaniasis in Syria requires the availability of an effective vaccine. DNA vaccine has recently provided a promising new approach. Nucleosomal core histones are very important for the parasite’s life, their genes showed a protective effect as DNA vaccines against infections by many species of Leishmania. The existence of these genes, like H2A gene, was not proved in L. tropica. Our study aimed to investigate the existence of H2A gene in a Syrian strain of L. tropica promastigotes and their expression. We isolated DNA and RNA from these parasites, synthesized cDNA, designed the necessary primers for gene`s amplification, and standardized the PCR conditions. This is the first study that proved the existence of this gene in a Syrian strain of L. tropica promastigotes. The sequence of the cDNA H2A gene was defined and published in Gene bank, its size was 399bp. Expression was also proved at the level of cDNA.
Keywords: Leishmania tropica; H2A gene; Syria
Abbreviations: cDNA: Complementary Deoxy Ribonucleic Acid; DNA: Deoxy Ribonucleic Acid; RNA: Ribonucleic Acid; L.: Leishmania
Introduction
Leishmaniases are infectious diseases caused by several species of the intracellular protozoan of Leishmania, which are transmitted by sandfly [1]. Leishmaniasis has different clinical manifestations, ranging from self-healing cutaneous ulcers to potentially fatal visceral infection [1]. These diseases represent a global public health problem, with an estimated prevalence of 12 million human beings infected. Syria is one of 9 countries have the majority of cutaneous Leishmaniasis cases in the world [2]. Current control is based on chemotherapeutic treatments which are expensive, toxic and associated with high relapse and resistance rates. Vaccination remains the best hope for control of all forms of the disease, so the development of a safe, effective and affordable antileishmanial vaccine is a critical global public-health priority. Immunization with naked DNA is a relatively new approach, which promises to revolutionize the prevention and treatment of infectious diseases [3]. Many studies have been made to find a protective antigen against leishmaniasis and many candidates have been studied for this aim, including histones. Histones are structural proteins found in the nucleus, where they play an important role in the organization and function of chromatin [4]. These highly conserved proteins that elicit strong immune responses are generally designated as panantigens [5]. Specific antibodies for parasite histones, obtained from dogs with visceral leishmaniasis, react against Leishmania H2A [6], H3 [7], H2B and H4 [8] but do not recognize mammalian histones. In the right conditions, these panantigens can provide the immunomodulatory properties needed for vaccine design [9]. So, these histones and their genes are good candidates for vaccine. H2A gene was sequenced in many species of Leshmania, but not in L. tropica, and their immunogenicity was tested [10,11]. We chose H2A gene, to design a DNA vaccine against the infection with Leishmania tropica which is responsible of the most cutaneous cases in Syria. The existence of H2A gene, wasn’t proved in L. trpoica. In this study; A methodical study was first made to know if this gene exists in the genome of a Syrian strain of Leishmania tropica, to prove if there is an expression of H2A Gene in these parasites, and to sequence this gene.
Materials and Methods
Primer design for PCR
Sequence of H2A gene in Leishmania tropica is not known so we made an alignment for the sequences of this gene in other species using CLC free workbench 7 and Vector NTI Express programs and the clustalw2 and emboss needle tools. Primers were designed using http://www.idtdna.com/calc/analyzer and http://www.geneinfinity.org/sms/sms_primanalysis.html tools. High purified Primers were ordered from VBC- Biotech, Vienna. Final sequences for primers were: (forward, 5'- ATTA GAATTC ATG GCT ACTCCTCGCAGCG -3'; reverse, 5’- CT TCTAGA CTAAGCGCTCGGTGTCGCCTTG -3’). Underlined are the ECORI and XBAI restriction sites included for direct cloning in the PCI mammalian expression vector (Promega, United States).
DNA extraction
DNA was extracted from the promastigotes of a Syrian strain Leishmania tropica (LCEBS, Damascus, Syria). DNA was isolated from Leishmania promastigotes, cultured on RPMI-1640 medium (Lonza, Switzerland) supported with Fetal bovine serum (Cytogen, GmbH, Germany), with DNA extraction kit (Thermo scientific, Lithuania) according to manufacturer’s instructions.
RNA isolation and cDNA synthesis
Total RNA was extracted from harvested Leishmania tropica promastigotes using TRI reagent (Sigma, St Louis, MO, USA). RNA was quantified by Nano Drop 1000 (Thermo Fisher Scientific MA, USA). RNA-integrity was checked by separating in 1.5% agarose gel. First strand cDNAs were synthesized using Revert Aid First Strand cDNA Synthesis kit (Thermo scientific, Lithuania) according to manufacturer’s instructions.
Polymerase chain reaction
DNA and cDNA of H2A gene were amplified by thermal cycler (Bio-Rad, USA) using PCR master mix (Thermo scientific, Lithuania) and the primers mentioned above. PCR protocol was optimized using gradient annealing temperatures. Reactions were performed in 25μl of a mixture containing 1µl of 10µM from each primer, 2, 5µl of template DNA or cDNA (110ng\µl), 12, 5µl of PCR master mix and 8µl of PCR water. Thermal cycling conditions were 95 °C for 5min followed by 35 cycles of 95 °C for 1min, 61.6 °C for 45sec and 72 °C for 1min finally 72 °C for 5 min. PCR products were loaded on a 2% agarose gel, stained with ethidium bromide, and visualized on a UV transilluminator.
Plasmid construction and purification
PCR product containing H2A coding sequence was purified using Gene Jet PCR Purification kit (Thermo scientific, Lithuania) according to manufacturer’s instructions. Purified cDNA and the PCI mammalian expression vector (Promega, United States) were double digested with ECORI plus XBAI. Digested cDNA was ligated with the digested PCI mammalian expression vector using t4 DNA ligase (Thermo scientific, Lithuania). PCI-H2A clones were transformed into E-coli TOP 10. Recombinant plasmid was extracted from colonies and purified using GF-1 plasmid DNA extraction kit (Vivantis, Malaysia).
Sequences analysis
Full-length double-stranded sequence analysis was performed on purified Recombinant plasmid PCI-H2A by sequencing, using T7 (forward) and PCR 3'(reverse) vector primers on automated sequencers (ABI PRISM Big Dye Terminator Cycle Sequencing Ready Reaction Kit, Perkin-Elmer, Foster City, USA).
Results and Discussion
Evaluation DNA and RNA quality and purity
Figure 1: Electrophoresis of extracted Leishmania tropica DNA: Lane 1 DNA ladder 1Kb, Lane 2,3 extracted Leishmania tropica DNA.
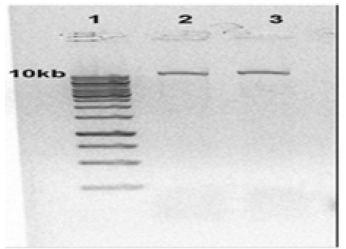
Figure 2: Electrophoresis of extracted Leishmania tropica RNA: Lane1 Leishmania tropica RNA, Lane 2 extracted Leishmania tropica DNA.
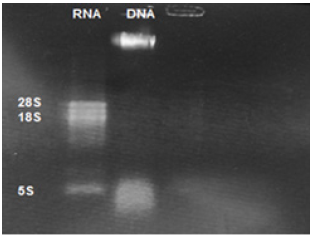
Extracted DNA electrophoresis on a 1% agarose gel only showed one band which proves that there isn't any degradation in the extracted DNA (Figure 1). The purity of the DNA was 1.8 which showed a high degree of purity. Extracted RNA electrophoresis on a 1.5% agarose gel showed a standard profile and there was no contamination with genomic DNA (Figure 2). The purity of the RNA was 2.1 which showed a sufficient degree of purity.
Polymerase chain reaction
Figure 3: Amplification products of Leishmania tropica H2A on 2% agarose gel electrophoresis stained with ethidium bromide. Lane 1 DNA ladder 50bp, lane 2 H2ADNA amplification product about 400bp.
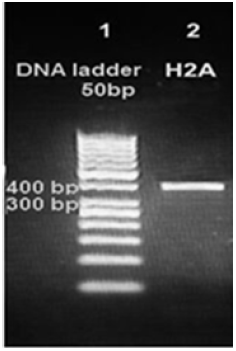
Figure 4: Amplification products of Leishmania tropica H2A on 2% agarose gel electrophoresis stained with ethidium bromide. Lane 1 DNA ladder 50bp, lane 2 H2AcDNA amplification product about 399bp.
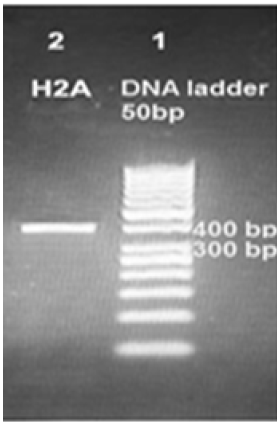
PCR protocol was optimized using gradient annealing temperatures and we chose the most suitable annealing temperature which was 61.6 °C. Gel electrophoresis of H2A PCR product on the level of DNA and cDNA showed only one band about 400bp which proves the specificity of our primers. So, we proved the existence of H2A gene in the genome of a Syrian strain of Leishmania tropica (Figure 3) and in the cDNA of this strain (Figure 4).
Cloning and sequencing of the H2A cDNA
Figure 5: Amplification products of Leishmania tropica H2A on 2% agarose gel electrophoresis stained with ethidium bromide. Lane 1 DNA ladder 50bp, lane 2 H2AcDNA amplification product about 399bp.

Figure 6: Nucleotide sequences of H2A cDNA for L.tropica, L.donovani, L.infantum, L.major and L.mexicana. The deduced amino acid sequence is indicated below the cDNA sequence.
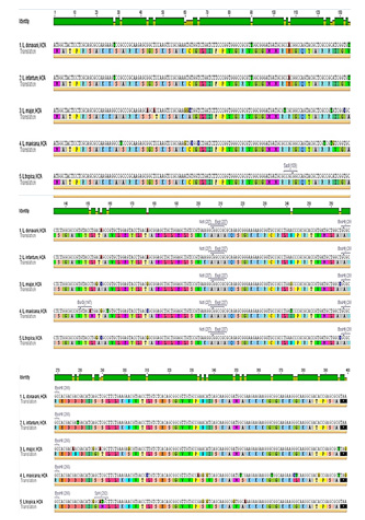
In an effort to clone and characterize the Leishmania tropica H2A gene, a cDNA clone was identified and sequenced. The sequence was submitted to the Gene bank under KT956066.1 Accession number. The complete nucleotide sequence of the 399bp cDNA insert, and the deduced amino acid sequence in comparison with other species were showed in (Figures 5) & (Figure 6), respectively. The deduced amino acid sequence predicts a protein containing 132 amino acids. The H2A predicted amino acid sequence was compared with the sequences of proteins coded by cDNA of H2A in other species of Leishmania such as L. infantum, L. major, L. donovania, L. mexicana and L. braziliensis using Vector NTI Program. This search revealed that H2A belongs to the histone H2A family. Interestingly, L. tropica H2A shows significant similarity with H2A histones of L. infantum (92.4%), L. major (91.7%), L. donovania (92.4%), L. mexicana (91.7%) and L. braziliensis (78%). The last high similarity shows that a good H2A DNA vaccine could protect from infection by the different species of Leishmania.
Conclusion
Our results proved the existence of H2A gene in the genomic DNA and the cDNA of a Syrian Leishmania tropica strain. We proved for the first time the existence of H2A gene in Leishmania tropica and defined the sequence of the cDNA of this gene and submitted it to the Gene bank under KT956067 accession number. PCI-H2A clones could now be tested to evaluate their immunogenicity as DNA vaccine against Leishmania species.
References
- Desjeux P (2004) Leishmaniasis: Current situation and new perspectives. Comp Immunol Microbiol Infect Dis 27: 305-318.
- Burden and distribution. World Health Organization, Geneva, Switzerland.
- Mutiso JM, Macharia JC, Kiio MN, Ichagichu JM, Rikoi H, et al. (2013) Development of Leishmania vaccines: Predicting the future from past and present experience. J Biomed Res 27: 85-102.
- Carneiro MW, Santos DM, Fukutani KF, Clarencio J, Miranda JC, et al. (2012) Vaccination with infantum chagasi nucleosomal histones confers protection against new world cutaneous leishmaniasis caused by leishmania braziliensis. PLoS One 7: e52296.
- Requena JM, Alonso C, Soto M, Requena JM, Alonso C, et al. (2000) Evolutionarily conserved proteins as prominent immunogens during leishmania infections. Parasitol Today 16: 246-250.
- Soto M, Requena JM, Quijada L, García M, Guzman F, et al. (1995) Mapping of the linear antigenic determinants from the Leishmania infantum histone H2A recognized by sera from dogs with leishmaniasis. Immunol Lett 48: 209-214.
- Soto M, Requena JM, Quijada L, Gomez LC, Guzman F, et al. (1996) Characterization of the antigenic determinants of the Leishmania infantum histone H3 recognized by antibodies elicited during canine visceral leishmaniasis. Clin Exp Immunol 106: 454-461.
- Soto M, Requena JM, Quijada L, Perez MJ, Nieto CG, et al. (1999) Antigenicity of the leishmania infantum histones H2B and H4 during canine viscerocutaneous leishmaniasis. Clin Exp Immunol 115: 342-349.
- Carrión J, Folgueira C, Alonso C (2009) Development of immunization strategies against leishmaniosis based on the leishmania histones pathoantigens. Procedia in Vaccinology 1: 101-103.
- Iborra S, Soto M, Carrión J, Alonso C, Requena JM (2004) Vaccination with a plasmid DNA cocktail encoding the nucleosomal histones of leishmania confers protection against murine cutaneous leishmaniosis. Vaccine 22: 3865-3876.
- Carrión J, Folgueira C, Alonso C (2008) Transitory or long-lasting immunity to leishmania major infection: The result of immunogenicity and multicomponent properties of histone DNA vaccines. Vaccine 26: 1155-1165.
© 2020 Franklin Binns. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






