- Submissions

Full Text
COJ Biomedical Science & Research
Effectiveness Of 10% Dextrose Solution for Acute Muscle Injury: Histology Study in Animal
Deviandri R*, Rasyid HN, Tiksnadi B and Mulyadi D
Department of Orthopaedics and Traumatology, Indonesia
*Corresponding author: Deviandri R, Department of Orthopaedics and Traumatology, Indonesia
Submission: March 12, 2020; Published: March 17, 2020

Volume1 Issue1March 2020
Abstract
Background: Muscle injury can be acute or repetitive. It can cause decrease muscle powers. Nowadays, treatment for acute muscle injury is developing rapidly, to shorter time of recovery. We need one modality that effective to fasten muscle healing. Dextrose have proliferant effect by promote growth factor in 12-lipoxygenase pathway. Dextrose 10% have proliferant effect without increase inflammation.
Objective: By using dextrose 10% injection intralesion were hoped fasten muscle healing process in acute muscle injury.
Method: This is experimental comparative research, was done on 20 rabbits that divided into two groups. After All of subject got muscle injury grade 2, The first group was administered with NaCl 0.9% as control, the second group with 10% dextrose injection intralesion. After one week, the subjects were sacrificed, and their gastrocnemius muscle were examined in pathology anatomy laboratory to see the level of myoblast.
Result: There was increased level of myoblast in dextrose 10% group than control. In conclusion, usage 10% dextrose solution fasten muscle healing process in muscle injury grade 2.
Keywords: Dextrose 10%; Muscle injury; Myoblast; Muscle healing; Prolotherapy
Introduction
Muscle injuries are the most frequent cause of physical incapacity in sports practice. It has been estimated that 30 to 50% of all sports-related injuries are caused by soft-tissue lesions. Muscle injuries can be caused by bruising, stretching, direct impact, punctured wounds, or excessive use in an exercise [1]. Muscle injury is a quite common injury that occured in acute, chronic or recurrent situation and leads to decreased performance. Initial phase of the treatment can be summarized as the “PRICE” protocol: Protect, Rest, Ice, Compression, and Elevation. Physiotherapy can be done by Trans Electrical Nerve Stimulation (TENS) or with a neodymium-YAG laser, followed by muscle exercise gradually. Surgery might be required in certain patients, such as in athletes with large intramuscular hematom, grade III muscle injury where the muscle cannot contract, grade III injuries when the rupture of the muscle more than 50%, and there is persistent pain in more than 6 months [2]. Normal movement usually after 4 weeks. But, the formation of connective tissue from myofibroblast will limit the movement and increase the risk of recurrence tear. This becomes a problem for professionals such as athletes who need a good and faster recovery. Therefore, a variety of methods and therapy modalities are developed to improve muscle injury recovery [3].
Repair of the muscle injury starts with two simultaneous processes that compete: Regeneration of the torn myofibrils and formation of connective scar tissue. Regeneration OS muscle cell was signed with myoblast cell in early phase. Several methods were offered to promote healing process of soft tissue such as prolotherapy. Prolotherapy is a nonsurgical regenerative injection technique that introduces small amounts of an irritant solution to the site of painful and degenerated tendon insertions (entheses), joints, ligaments, and in adjacent joint spaces during several treatment sessions to promote growth of normal cells and tissues. Irritant solutions most often contain dextrose (d-glucose), a natural form of glucose normally found in the body, but may also contain combinations of polidocanol, manganese, zinc, human growth hormone, pumice, ozone, glycerin, or phenol [4]. Dextrose solution increased 12-lipoxygenase pathway (12-LO) on arachidonic acid metabolism which has the effect of angiogenesis and increase the level of growth factors on muscle cell [5].
Methods
This study is a comparative experimental study using New Zealand rabbits. The first group which is administered saline solution as control group, the second group which is administered 10% dextrose.
Inclusion criteria were New Zealand rabbits which meets the following requirements:
- Male, age 7 months
- Weight 1000-1500 grams
- In healthy conditions
Exclusion criteria were as follows:
- Infected during the study
- Death during the study
This research was conducted with several stages as follows:
- Preparation of rabbits as the experiment sample
- Male New Zealand rabbits aged 7 months with weight of 1000-1500 grams
- Quarantined for 7 days for adaptation of new environment before being given the treatment.
- The rabbits were randomized into 2 groups, each group consisting of 10 rabbits, and were put into a cage with certain treatment.
- Treatment in rabbit’s gastrocnemius muscle with grade 2 muscle injury.
- The sample rabbits were sedated with HCl ketamine intramuscularly.
- The hair of rabbit’s lower limbs was shaved.
- Aseptic and antiseptic procedure with 70% alcohol and 10% povidone iodine was done.
- Muscle injury grade 2 was performed by incise gastrocnemius muscle in 30 percent of muscle diameter, then close the wound
- The group then divided into two groups where the first group was given saline solution and the second group was given 10% dextrose solution.
- After receiving treatment, the leg was immobilized by a circular cast.
- Maintenance of rabbits
- During maintenance, the rabbits were given the same food and drink.
- Post experiment
- After 1 week, the sample material of injured muscle will be taken.
- The sample material with a size of 0.5x0.5x0.5cm was put into a tube containing formalin.
- The sample is then brought to the Laboratory of Pathology for preparations.
- Immunohistochemistry examination
- The sample added with rmyoD1 reagent
- Under the microscope CX-21, the intensity and distribution of MyoD1 binding with myoblast cells in each preparat will be counted.
- Analysis of data and statistics
- Data was processed with SPSS 25.0 for Windows.
- Then data was analyzed with univariate analysis.
- Then the normality of the data is tested with the Shapiro-Wilk test.
- Then proceeded with the homogenity test using Levene test.
- Then proceeded with T-Test or Mann Whitney test
Result
This research was conducted using experimental animals of 20 male New Zealand rabbits which are divided into 2 groups. The first group was treated with saline solution as control group, the second group was treated with 10% dextrose. One week after treatment, samples were taken from the rabbit’s gastrocnemius muscle with 0.5x0.5x0.5cm lesion and immunohistochemical examination using myoD1 reagent was done, and the myoblast distribution was calculated with Histoscore method by scoring the intensity and myoblast cells distribution [6].
Myoblast cell intensity with MyoD1 divided into three scoring [6]:
- (+) Weak with score of 1,
- (+) Moderate with score of 2, and
- (+) strong with score of 3.
Myoblast cell distribution was divided into four scoring [6]:
- <20% with a score of 1,
- 20-50% with a score of 2,
- 51-80% with a score of 3, and
- >80% with a score of 4.
The immunohistochemical examination results were scored and compared between two groups, where the value of the score is based on Hscore scoring system with formula:
Hscore=(intensity+1) x myoblast distribution [6]
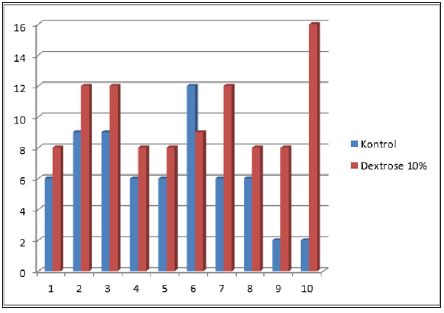
Assessment was given in the form of ordinal scale scores with a total score ranging from 2-16. Figure 1-3 shows a picture of the myoblast cell anatomical pathology binded to the reagent myoD1 at various intensities. Research results for the three groups of rabbits were descriptively shown in the Figure 4. Figure 4 illustrates the scoring of myoblast from muscle healing in rabbits. Based on these data it can be seen that the lowest score in the dextrose group is 8, the highest score is 16 and the mean score is 10.1; lowest score at control group is 2, the highest score is 12 and the mean score is 6.4; Indicating that 10% dextrose group mean score was higher than control group. Then the Mann Whitney test was done to see whether there is significant difference in the number of myoblasts cells in the rabbit’s muscles between the dextrose group and the control group. Based on the statistical calculation, it was obtained that Z value -2,432 with P value 0.015. This statistical analysis showed that the value of P<0.05. Therefore, we can conclude that there are differences in the number of myoblast cells in the rabbit’s muscles in the 10% dextrose group towards the control group.
Figure 1: Picture of myoblast cells that bind MyoD1 on strong intensity.
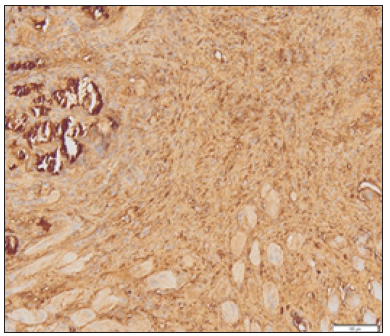
Figure 2: Picture of myoblast cells that bind MyoD1 at moderate intensity.
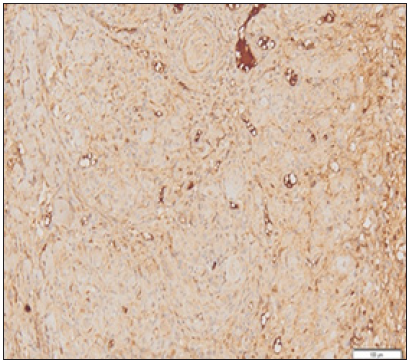
Figure 3: Picture of myoblast cells that bind MyoD1 on weak intensity.
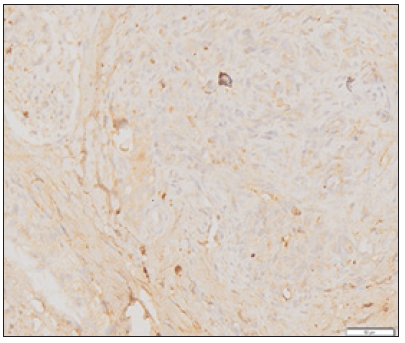
Figure 4: Hscore scoring results of myoblast cells distribution in each rabbit.
Discussion
These results show that administration of intralesional injection of dextrose 10% on the injured muscle is proved effective in muscle healing by increasing the number of myoblast cells in the muscle (p<0.05). Increased number of myoblast cells in the 10% dextrose group proved that dextrose solution has proliferant effect to the soft tissue. Dextrose can provide a stimulant effect to increase the level of growth factor that plays an important role in muscle cell migration and proliferation [4]. The cells will produce growth factors in a few minutes to a few hours when exposed with dextrose in concentrations above 0.6% (the normal cell glucose concentration is 0.1%) [7]. Extracellular hyperglycemic condition will increase 12-lipoxygenase pathway (12-LO) on arachidonic acid metabolism which has the effect of angiogenesis and increasing the level of growth factors on muscle cells [4]. These growth factors are Platelet Derived Growth factors, transforming growth factors beta, Epidermal Growth factors, Basic Fibroblast Growth factors, Insulin Like Growth factors and Connective Tissue Growth factors. All these factors have been identified as the key to stimulate the healing of muscle injuries [8,9].
The advantage used of 10% dextrose solution is it will induce proliferation without an increase in inflammatory reactions, making it suitable to be used in cases of acute muscle injury [7]. Dextrose Prolotherapy is presumed to work by several mechanisms including a direct, an osmotic, and inflammatory growth effect. Dextrose injections below a 10% solution directly stimulate proliferation of cells and tissue without causing a histological inflammatory reaction. When dextrose is injected in greater than 10% solution it is presumed to be causing an osmotic (concentrated) gradient outside of the cells where it is injected. This causes some cells to lose water and lyse with the net effect being an influx of growth factors and inflammatory cells [10,11]. Dextrose is an ideal proliferant because it is water soluble and a normal component of blood chemistry, which can be injected safely into multiple areas and in large quantity. The presumed net result is the deposition of new collagen into injured structures, such as ligaments and tendons [7].
Increasing number of myoblast cells in this study assessed based on scoring system of HScore. Hscore scoring system evaluates two important variables shown in healing of the injured muscle, the intensity and distribution of myoblast cells. Assessment was carried out by a specialist consultant of anatomical pathology to minimize the possibility of bias [12]. The weakness in this study is the difficulties in terms of uniformity of rabbit’s gastrocnemius muscle diameter, so there is a possibility of bias at the time of sampling for immunohistochemical examination.
Conclusion
A. 10% dextrose intralesional injection on injured muscle grade 1 may increase the number of myoblast cells.
B. To improve healing process in acute muscle injury, 10% dextrose solution can be an option.
References
- Fernandes TL, Pedrinelli A, Hernandez AJ (2015) Muscle injury-physiopathology, diagnosis, treatment and clinical presentation. Rev Bras Ortop 46(3): 247-255.
- Kucera KL, Lipscomb HJ, Roos KG, Dement JM, Hootman JM (2018) Work-related injury and management strategies among certified athletic trainers. J Athl Train 53(6): 606-618.
- Harmon KG, Drezner JA, Gammons M, Guskiewicz KM, Halstead M, et al. (2013) American medical society for sports medicine position statement: Concussion in sport. Br J Sports Med 47(1): 15-26.
- Hauser R, Lackner JB, Matias DS, Harris DK (2016) A systematic review of dextrose prolotherapy for chronic musculoskeletal pain. Clin Med Insights Arthritis Musculoskelet Disord 9: 139-159.
- Sangho O, Ettema AM, Zhao C, Zobitz ME, Wold LE (2008) Dextrose-induced subsynovial connective tissue fibrosis in the rabbit carpal tunnel: A potential model to study carpal tunnel syndrome? Hand 3(1): 34-40.
- Warrington R, Watson W, Kim H, Antonetti F (2011) An introduction to immunology and immunopathology. Allergy Asthma Clin Immunol 7(Suppl 1): S1.
- Hauser R, Hauser M, Baird N (2011) Evidence based of dextrose prolotherapy for musculoskeletal pain: A scientific literature review. Journal of Prolotherapy 3(4): 765-789.
- Topol GA, Podesta LA, Reeves KD, Raya MF, Fullerton BD, et al. (2011) Hyperosmolar dextrose injection for recalcitrant Osgood Schlatter disease. Pediatrics 128(5): e1121-8.
- Jarvinen T, Järvinen TL, Kääriäinen M, Aärimaa V, Vaittinen S, et al. (2007) Muscle injuries: Optimising recovery. Best Pract Res Clin Rheumatology 21(2): 317-331.
- Jensen K, Rabago DP, Best TM, Patterson JJ, Vanderby R, et al. (2008) Response of knee ligaments to prolotherapy in a rat injury model. Am J Sports Med 36(7): 1347-1357.
- Jensen K, Rabago DP, Best TM, Patterson JJ, Vanderby R, et al. (2008) Early inflammatory response of knee ligaments to prolotherapy in a rat model. J Orthop Res 26(6): 816-823.
- Dias P, Parham DM, Shapiro DN, Tapscott SJ, Houghton PJ (1992) Monoclonal antibodies to the myogenic regulatory protein MyoD1: Epitope mapping and diagnostic utility. Cancer Res 52(23): 6431-6439.
© 2020 Deviandri R. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






