- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Using In-Silico Molecular Docking as a Tool to Justify the Anti-Typhoid Property of Terminalia Chebula
Karishma Kundu* and Dorothy Pushparani
Karishma Kundu,Department of Chemistry, Food Chemistry and Food Processing, Loyola College, Chennai- 600034, Tamil Nadu, India
*Corresponding author: Lieshchova MA, Dnipro State Agrarian and Economic, University, Sergii Efremov, Ukraine Mylostyvyi RV, Dnipro State Agrarian and Economic, University, Sergii Efremov, Ukraine
Submission: February 15, 2023; Published: March 09, 2023

ISSN 2578-0190 Volume6 issues4
Abstract
In this study, the anti-typhoid activity of Terminalia chebula (Myrobalan), referred to as the ‘King of Medicine in Ayurveda, was verified using a promising, novel method known as In-silico molecular docking technique. The dried fruits of the myrobalan were powdered, extracted, and observed for secondary metabolites, followed by the anti-larvicidal activity towards Culex quinquefasciatus (mosquitoes) and the anti-salmonella activity of Salmonella Typhimurium ATCC 14028 (causing typhoid fever in mice). The ethyl acetate extracts actively showed inhibition in growth in the experimental setup. Further, to justify the anti-salmonella activity, a GC-MS was performed for better profiling of the ethyl acetate extract of myrobalan in terms of active compounds. A complex mixture of 9 bioactive compounds, many of them were present in traces; however, Borane-methyl Sulfide complex (40.084%), Methyl hydrogen Disulfide (52.779%), Disulfide, methyl (Methylthio) methyl (19.342%), Ethene, (Methylsulfinyl)-(16.957%) were pinned as most versatile compounds. The compounds with higher retention time in GC-MS were selected for the molecular docking studies. The In-silico docking confirmed the role of the bioactive molecule present in Terminalia chebula as a potential compound for treating typhoid fever by successfully outlining the active bonding between the active compound with the 2YM0 protein receptor from Salmonella typhimurium.
Keywords: In-silico molecular docking; typhoid fever; Terminalia chebula; Salmonella typhimurium; Culex quinquefasciatus; metabolite profiling
Introduction
Typhoid is a bacterial enteric infection caused by Salmonella enterica serovar typhi (Salmonella typhi; S. typhi [1]. It is one of the bacteria that cause acute febrile sickness that is most common in underdeveloped countries. Inadequate sanitation in these nations allows for easy contamination of food and water with human waste, making typhoid fever an international public health concern [2,3]. Countries in South Asia, the Middle East, Central Africa, and South America have all been reported to have high endemic illness rates and be experiencing typhoid fever epidemics. Worldwide, typhoid is responsible for between 128,000 and 161,000 annual deaths [4]. Typhoid affects an estimated 11-20 million individuals each year. India has estimated 4.5 million cases of typhoid fever per year, with roughly 9000 deaths (0.2% case fatality rate). This equates to an annual incidence rate of 297-494 per 100,000 people [5].
Antibiotics effectively treat typhoid fever, provided a correct diagnosis is made within the first few days of symptoms appearing. However, the increasing prevalence of MDR S. typhi strains hinders effective treatment and may increase the incidence of sequelae and disease severity [4]. S. typhi infections are typically treated with cephalosporins, azithromycin, ampicillin, chloramphenicol, and cotrimoxazole. Ofloxacin and ciprofloxacin, two fluoroquinolones, have been increasingly used in recent years because of the widespread prevalence of multidrugresistant bacteria [6,7]. Typhoid vaccinations have made significant strides recently, representing a powerful resource for the international community’s fight against the disease. The World Health Organization (WHO) recommends routinely administering Typhoid Conjugate Vaccine (TCV) as well as conducting targeted catch-up vaccination to reduce the prevalence of typhoid fever [4,8]. Yet, the existing diagnostic techniques can lead to under or over-evaluation of the condition, delaying therapy or causing improper, excessive antibiotic usage, increasing the probability of bacterial resistance [9].
Treatment with medicinal plants is more common than conventional medications because of their low cost and wide availability. Phytoconstituents found in nature have chemical characteristics like those of artificial antibiotics. Due to documented problems with monotherapy antibiotic efficacy, assessing plant biological characteristics is crucial. Tulsi, also known as Ocimum sanctum, is an herb native to India that has been shown to have intense antibacterial activity against S. typhi [9]. A combination of chloramphenicol and trimethoprim with O. sanctum leaf extract is highly effective against S. typhi, as shown in in vitro research [10]. The inhibitory activity of acetone mango leaf extract (10-50g/ml) against multidrug-resistant S. typhi was also demonstrated [11]. Leaf extracts from Cymbogogon citratus, Carica papaya, and Zea mays silk in methanol have been shown to have a MIC against S. typhi between 0.02-0.06g/ml [12]. Although numerous studies have demonstrated the efficacy of various natural extracts against S. typhi in test tubes, little is known about their absorption, pharmacokinetics and pharmacodynamics, antibacterial interaction, or safety profile in humans [12,13].
Terminalia chebula contains tannins (32-34%). The most abundant phytoconstituents in this fruit are phenolic compounds, alkaloids, glycosides, flavonoids, saponins, quinine, steroids, etc. [8- 16]. The various studies conducted provides the evidence for the versatility of the Terminalia chebula, revealing its diverse range of properties such as antibacterial activity [17]; anti-mutagenic, radio-protective, chemo-preventive activity, anti-anaphylaxis [18]; immunomodulatory activity [19]; anti-carcinogenic activity [20]; antifungal activity, antiviral activity [21]; hepato-protective activity, cardioprotective activity, cytoprotective activity, anti-diabetic, reno-protective activity [22]; anti-inflammatory and anti-arthritis property, gastrointestinal motility improving, anti-ulcerogenic activity, anti-plasmodia activity, anti-caries activity, wound healing activity, anti-allergic activity, anti-protozoal activity, anti-aging [23]; anti-Salmonella activity [24]; antioxidant and free radical scavenging activity [25]; hypo-cholesterelomic activity against cholesterol-induced hypercholesterolemia and atherosclerosis [26]; anti-diarrheal [27]; anti- HIV activity [28]; hypo-lipidemic activity and nephron-protective activity. In addition, grampositive and gram-negative microorganisms are susceptible to the fruit’s broad spectrum of antimicrobial capabilities [29]. Our study attempted to better establish the interaction between the secondary metabolites or compounds found in the myrobalan fruit (Terminalia chebula) and the 2YM0 protein (Truncated SipD from ) in preventing typhoid fever.
Results
Phytochemical analysis
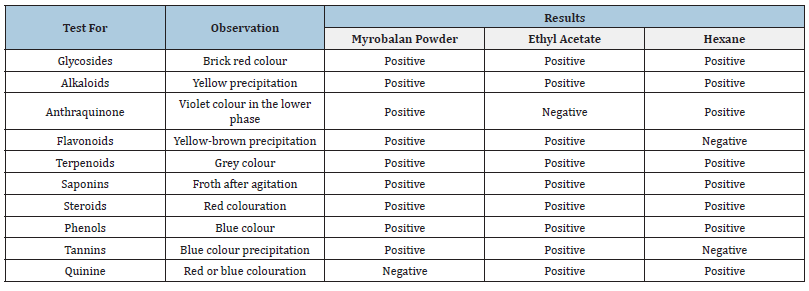
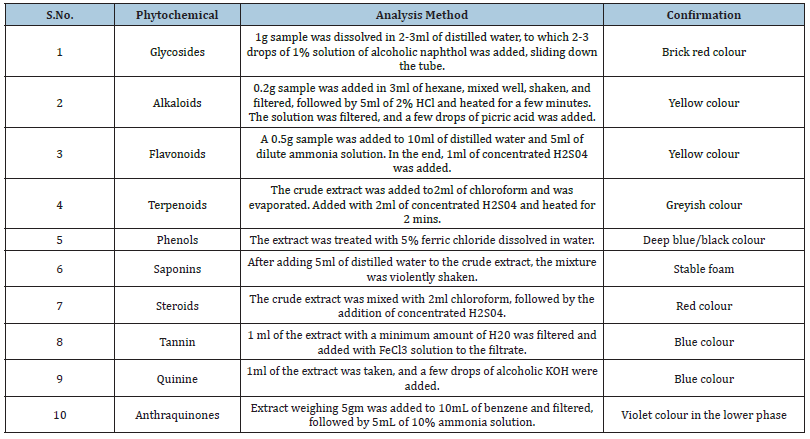
The fruit powder showed the presence of most of the phytochemicals except quinine. The crude extract of hexane shows the presence of fewer phytochemicals than the ethyl acetate extract (Table 1).
Table 1:Qualitative phytochemical analysis of myrobalan fruit powder and two extracts.
Larvicidal assay
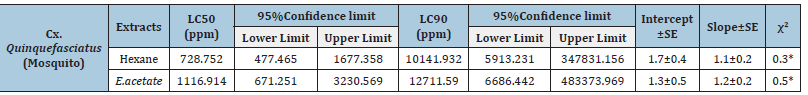
The results highlighted the good larvicidal activity of the two extracts, Hexane, and ethyl Acetate of Myrobalan powder, against the larvae of Culex quinquefasciatus. At 1116.914ppm and 12711.590ppm for 24 hours, respectively, the Ethyl Acetate extract had the most vital larvicidal activity (Table 2).
Table 2:The effects of Terminalia chebula crude extracts on Culex quinquefasciatus larvae at various doses.
Anti-salmonella activity
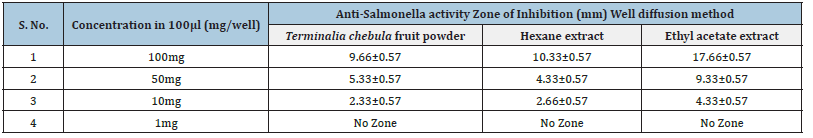
The Terminalia chebula fruit powder at a concentration of 1mg/100μl showed no zone of inhibition. At a concentration of 10mg/100μl, the zone of inhibitions was 2.33±0.57, and at a concentration of 50mg/100 μl, the zone of inhibitions was 5.33±0.57. The concentration of 100mg/100μl showed a zone of inhibition of 9.66±0.57. Results conclude that the zone of inhibition of Ethyl Acetate extract Terminalia chebula fruit powder is higher than Hexane extract at the same concentrations. The positive control was 2.5mg Chloramphenicol in 100μl, and the negative control was distilled water (Table 3).
Table 3:The anti-Salmonella activity of Terminalia chebula fruit powder v/s Hexane extracts v/s Ethyl Acetate extract using agar well diffusion method (N= 3).
GCMS analysis
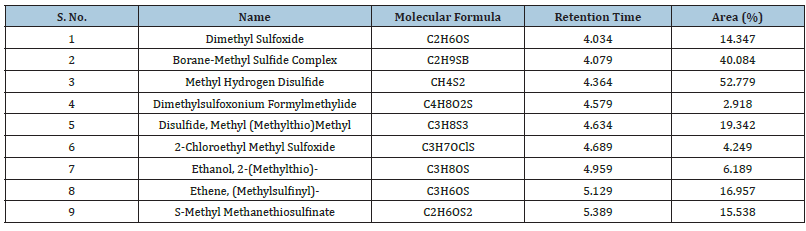
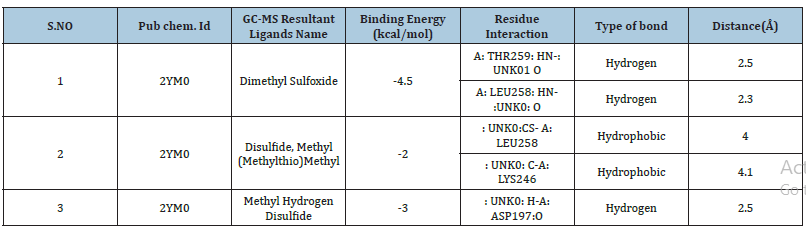
The chemical constituents in the ethyl acetate extract showed a complex mixture of 9 bioactive compounds; many were present in trace amounts. On the other hand, Boranemethyl Sulfide complex (40.084%), Methyl hydrogen Disulfide (52.779%), Disulfide, methyl (Methylthio) methyl (19.342%), Ethene, (Methylsulfinyl)-(16.957%) were pinned as the versatile compounds (Table 4).
Table 4:Qualitative Phytochemical Analysis of Myrobalan fruit powder and two extracts.
Molecular docking
Molecular docking was carried out against receptor protein 2YM0 (from Salmonella typhimurium) and the GC-MS resultant ligands, namely (1) Dimethyl Sulfoxide, (2) Disulfide, Methyl (Methylthio) Methyl, (3) Methyl Hydrogen Disulfide. The results showed that all three ligands could intensely occupy the active location of 2YM0 with their binding energies. The compound (1) Dimethyl Sulfoxide exhibited a high value of -4.5 (Kcal/mol) binding-energy. H-bonds interaction between this ligand and 2YM0 protein. Figures 1-9 depict the H-bond interactions between ligands and receptors, as well as H-bond interactions on the receptor side and their 2D-ligand-receptor H-bond interaction (Table 5).
Figure 1:Ligand-receptor hydrogen bond interactions of Dimethyl Sulfoxide.
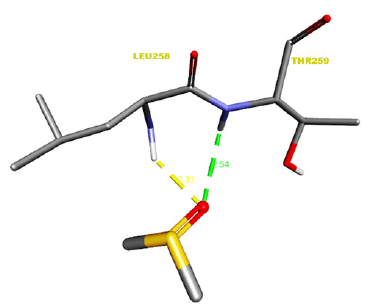
Figure 2:Receptor side hydrogen bond interactions of Dimethyl Sulfoxide.
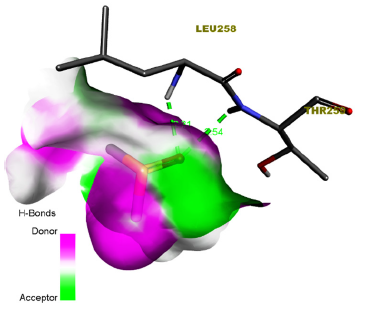
Figure 3:Ligand-receptor hydrogen bond interaction of Dimethyl Sulfoxide.
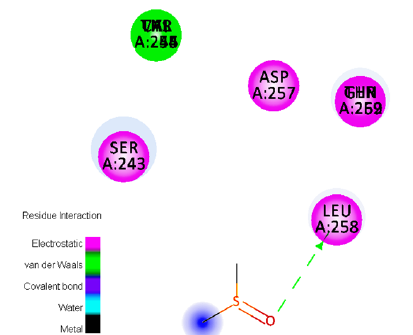
Figure 4:Ligand-receptor hydrogen bond interactions of Disulfide, Methyl (Methylthio)Methyl.
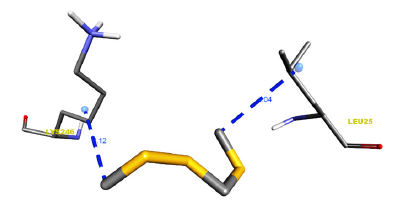
Figure 5:Receptor side hydrogen bond interactions of Disulfide, Methyl (Methylthio)Methyl.
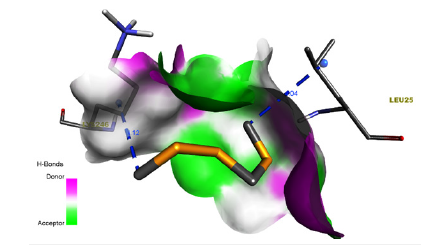
Figure 6:Ligand-receptor hydrogen bond interaction of Disulfide, Methyl (Methylthio)Methyl..
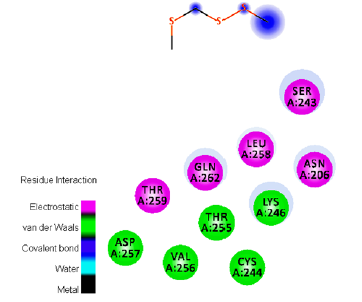
Figure 7:Ligand-receptor hydrogen bond interactions of Methyl Hydrogen Disulfide.
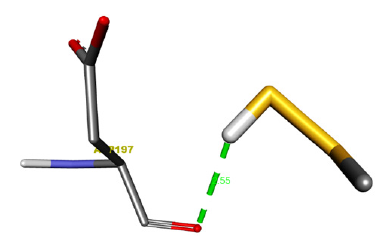
Figure 8:Receptor side hydrogen bond interactions of Methyl Hydrogen Disulfide
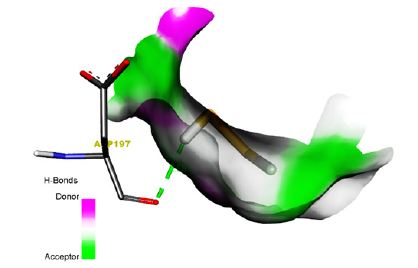
Figure 9:Ligand-receptor hydrogen bond interaction of Methyl Hydrogen Disulfide.
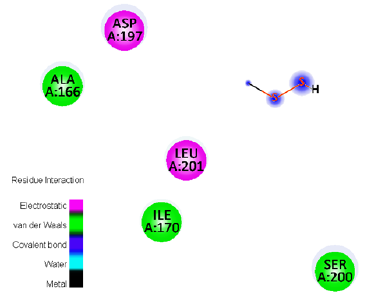
Table 5:The interaction between GC-MS resulting Ligands and the 2YM0 receptor residue.
Materials and Methods
Sample harvesting
Terminalia chebula dried fruits were purchased from a local market in Chittaranjan Park, New Delhi, India. Each fruit was thoroughly checked for its quality. The required amount of fruit was de-seeded, initially broken using a mortar pestle, and later powdered using a powerful blender. A measured amount of fruit powder was extracted using hexane and ethyl acetate. The extraction process was run for two days at 70 ̊C. The two extracts were evaporated and stored for future use [30].
Phytochemical analysis/
The phytochemical profiling was carried out for various metabolites [12,31-36] (Table 6).
Table 6:The phytochemical profiling was carried out for various metabolites.
Larvicidal bioassay
The Bioassay was carried out following WHO 2005 guidelines, with minor modifications. Third instar larvae of Culex quinquefasciatus, 20 in number, were moved into 100ml containers. 500ppm of hexane and ethyl acetate extract was made with 1.0% DMSO. As a control, 1% DMSO in water was used. After the period of 24h, the mortality rate was observed. The larvae were accounted dead after showing no mobility when touching a needle [33,37,38].
Anti-salmonella assay
The agar well diffusion method investigated the antimicrobial activity of gram-ve, pathogenic Salmonella typhimurium ATCC 14028. The freshly made agar was allowed to be set, and after solidification, wells were made, i.e., four wells were cut on each agar plate using an 8mm well cutter. The culture of the above bacteria was swabbed into a 90mm agar plate. The diameter of the zone of inhibition formed around the well was measured after 72 hours of incubation at 37 degrees Celsius. As a control, distilled water was used [24,39].
GCMS analysis
The spectra of secondary metabolites were observed for the ethyl acetate extract using GC–MS (SHIMADZU QP2010). GC specifications: injector temperature -260 ˚C, column oven temperature -60 ˚C, column flow was 1mL/min, injection mode in split with a ratio of 10:1, flow control mode in linear velocity, Helium (99.99%) purity was used as carrier gas. The MS specifications were, Ion source temperature at 230 ˚C, interface temperature at 230 ˚C, solvent cut time was 5min, event time was 0.5s, Ionization (EI) was at 70EV, the start time was 5min and end time was 35min, scan range from 50 to 600Da, etc. Compounds were recognized by their twin GC retention times [40,41].
Molecular docking analysis/
The docking program Auto Dock via 4.2 was used to select the possible binding mode between the GC-MS resultant ligands and 2YM0 receptor protein. Software Chem Draw 12.0 was used to draw ligand structures. After minimizing energy for these 2D compound structures, they were changed into Protein Data Bank (PDB) format, which was further transmuted into the PDBQT structure. Auto dock Tools prepared the 2YM0 protein structure by adding required H-atoms applying solvation parameters, and Kollman united atom charges. Ligand structures were created by combining non-polar H-atoms, Gasteiger partial charges, and defining rotatable bonds. An Auto grid was used to create grid boxes with a maximum size of 108 x 112 x 69. The grid box was designed with a grid spacing of 0.492 at the binding site for 2YM0 at the midpoint of the proteins with x (-3.005), y (19.46), and z (-21.473) coordinates. Discoverystudio visualizes 4.0 was used to create a 2D graphic representation of protein-ligand interaction [42,43].
Conclusion
The Terminalia chebula (myrobalan) powder is shown to have a wide range of secondary metabolites in the hexane and the ethyl acetate extract. Notably, the anti-larvicidal and anti-salmonella activity test results exhibited the property of inhibiting the growth of Culex quinquefasciatus (mosquito) and Salmonella typhimurium ATCC 14028 for both Terminalia fruit extracts; however, the ethyl acetate extract showed better outcomes than the hexane extract. In addition, the GCMS analysis of the ethyl acetate extract revealed the presence of 9 bioactive components, with the Borane-methyl Sulfide complex, Methyl hydrogen Disulfide, Disulfide, methyl (Methylthio) methyl, Ethene, (Methylsulfinyl) compound having the highest retention time [43-48]. Lastly, the molecular docking results revealed that the bioactive molecule Dimethyl Sulfoxide present in Terminalia chebula is the most potential bioactive compound for treating typhoid fever.
Funding
This study was funded by Tamil Nadu State Council for Science and Technology (TNSCST) under Student Project Scheme 2019 code PS-025 and was awarded “Best Oral Presentation” in the Physical Sciences category at the Seminar cum Exhibition, organized by Tamil Nadu State Council for Science and Technology (TNSCST) at Kalasalingam Academy of Research and Education, Krishnankoil, Tamil Nadu - 626126 during 19-20 July 2019.
References
- Raffatellu M, Wilson RP, Winter SE, Bäumler AJ (2008) Clinical pathogenesis of typhoid fever. J Infect Developing Countries 2(4): 260-266.
- Mather RG, Hopkins H, Parry CM, Dittrich S (2019) Redefining typhoid diagnosis: What would an improved test need to look like? BMJ Glob Health 4(5): 1-9.
- Radhakrishnan A, Als D, Mintz ED, Crump JA, Stanaway J, et al. (2018) Introductory article on global burden and epidemiology of typhoid fever. American Journal of Tropical Medicine and Hygiene 99(3): 4-9.
- Kim CL, Cruz E, Vannice KS, Tadesse BT, Owusu DE, et al. (2022) The burden of typhoid fever in sub-Saharan Africa: A perspective. Res Rep Trop Med 13: 1-9.
- Cao Y, Karthikeyan AS, Ramanujam K, Raju R, Krishna S, et al. (2021) Geographic pattern of typhoid fever in India: A model-based estimate of cohort and surveillance data. J Infect Dis 224: S475-S483.
- Dewan E, Verma V (2019) Antimicrobial susceptibility trends in salmonella enterica isolates - A 6-year study from a tertiary care hospital in North India. J Evol Med Dent Sci 8(42): 3119-3124.
- Harish BN, Menezes GA (2011) Antimicrobial resistance in typhoidal salmonellae. Indian J Med Microbiol 29(3): 223-229.
- Syed KA, Saluja T, Cho H, Hsiao A, Shaikh H, et al. (2020) Review on the recent advances on typhoid vaccine development and challenges ahead. Clinical Infectious Diseases 71(2): S141-S150.
- Anand U, Jacobo HN, Altemimi A, Lakhssassi N (2019) A comprehensive review on medicinal plants as antimicrobial therapeutics: Potential avenues of biocompatible drug discovery. Metabolites 9(11): 258.
- Mandal S, Deb M, Kumar PN (2012) Enhancing chloramphenicol and trimethoprim in vitro activity by Ocimum sanctum Linn. (Lamiaceae) leaf extract against Salmonella enterica serovar typhi. Asian Pac J Trop Med 5(3): 220-224.
- Safdar MN, Kausar T, Jabbar S, Mumtaz A, Ahad K, et al. (2017) Extraction and quantification of polyphenols from kinnow (Citrus reticulate L.) peel using ultrasound and maceration techniques. J Food Drug Anal 25: 488-500.
- Baliah NT, Muthulakshmi P, Celestin SP, Lega P (2008) Green synthesis and characterization of nanocomposites. Journal Green Synthesis and Characterization of Nanocomposites 5(12): 179-186.
- Pragasam B, Bakthavatchalam AK, Ralph YD (2018) Typhoid fever: Issues in laboratory detection, treatment options & concerns in management in developing countries. Future Sci OA 4(6).
- Moretta A, Scieuzo C, Petrone AM, Salvia R, Manniello MD, et al. (2021) Antimicrobial peptides: A new hope in biomedical and pharmaceutical fields. Front Cell Infect Microbiol 11: 668632.
- Thakur M (2008) Physiochemical evaluation of Terminalia chebula J Non Timber Forest Prod 15: 37-42.
- Tubtimdee C, Shotipruk A (2011) Extraction of phenolics from Terminalia chebula Retz with water-ethanol and water-propylene glycol and sugaring-out concentration of extracts. Sep Purif Technol 77(3): 339-346.
- Sato Y, Oketani H, Singyouchi K, Ohtsubo T, Kihara M (1997) Extraction and purification of effective antimicrobial constituents of Terminalia chebula against methicillin-resistant Staphylococcus aureus. Biological and Pharmaceutical Bulletin 20(4): 401-404.
- Shin TY, Jeong HJ, Kim DK, Kim SH, Lee JK, et al. (2001) Inhibitory action of water-soluble fraction of Terminalia chebula on systemic and local anaphylaxis, Journal of Ethnopharmacology 74(2): 133-40.
- Malekzadeh F, Ehsanifar H, Shahamat M, Levin M, Colwell RR (2001) Antibacterial activity of black myrobalan (Terminalia chebula Retz) against helicobacter pylori, International Journal of Antimicrobial Agents 18(1): 85-88.
- Saleem A, Husheem M, Härkö NB, Pihlaja P (2002) Inhibition of cancer cell growth by crude extract and the phenolics of Terminalia chebula retz. Fruit 81(3): 327-336.
- Yukawa TA, Kurokawa M, Sato H, Yoshida Y, Kageyama S (1996) Prophylactic treatment of cytomegalovirus infection with traditional herbs. Antiviral Research 32(2): 63-70.
- Sabu MC, Kuttan R (2002) Anti-diabetic activity of medicinal plants and its relationship with their antioxidant property. Journal of Ethnopharmacology 82(2): 155-160.
- Mahesh R, Bhuvana S, Begum VM (2009) Effect of Terminalia chebula aqueous extract on oxidative stress and antioxidant status in the liver and kidney of young and aged rats. Cell Biochem Funct 27: 358-363.
- Hayat KK, Jain SK (2009) Regular intake of Terminalia chebula can reduce the risk of getting typhoid fever. Open Access Library Journal 3(3).
- Hazra B, Sarkar R, Biswas S, Mandal N (2010) Comparative study of the antioxidant and reactive oxygen species scavenging properties in the extracts of the fruits of Terminalia chebula, Terminalia belerica and Emblica officinalis, BMC Complementary and Alternative Medicine 10: 20.
- Bag A, Bhattacharyya SK, Chattopadhyay RR (2013) The development of Terminalia chebula Retz. (Combretaceae) in clinical research. Asian Pac J Trop Biomed 3(3): 244-252.
- Fu K, Xu M, Zhou Y, Li X, Wang Z, et al. (2020) The Status quo and way forwards on the development of Tibetan medicine and the pharmacological research of Tibetan Materia Medica. Pharmacol Res 155: 104688.
- Kolla JN, Kulkarni NM, Kura RR, Theepireddy SK (2017) Terminalia chebula Retz an important medicinal plant. Herba Polonica 63(4): 45-56.
- Naher S, Ferdous B, Datta T, Rashid F, Tasnim N, et al. (2013) Ayurvedic influences in folk medicine: A case study of a folk medicinal practitioner of Jhalokathi in Barisal district, Bangladesh. Journal of Sustainable Agriculture 7: 295-305.
- Mamatha C, Hena J, Vimalin (2015) Phytochemical analysis of Terminalia chebula and its activity against Acinetobacter baumanii. Microbiology and Bio Techniques (SIRJ-MBT) 2.
- Lim YP, Pang SF, Yusoff MM, Abdul M, Gimbun SK (2019) Correlation between the extraction yield of Mangifera to the antioxidant activity, total phenolic and total flavonoid content of Phaleria macrocarpa fruits. J Appl Res Med Aromat Plants 14: 100224.
- Pal Singh (2010) Pharmacognostical Evaluation of Terminalia Chebula fruits on different market samples, Article in International Journal of Chem Tech Research 2(1): 57-61.
- Solomon CU, Uche A, Ifeanyi O (2013) Preliminary phytochemical screening of different solvent extracts of stems bark and roots of Dennetia tripetala G Baker. Pelagia Research Library Asian Journal of Plant Science and Research 3(3): 10-13.
- Wadood A (2013) Phytochemical analysis of medicinal plants occurring in local area of mardan. Biochem Anal Biochem 2: 4.
- Wijesundara SA, Kannangara BT, Abeywickrama K (2016) Antifungal activity of Croton aromaticus L in vitro, against post-harvest fungal pathogens isolated from tropical fruits. J Agric Sci (Belihuloya) 11(2): 105-117.
- Yadav R, Agarwala M (2011) Phytochemical analysis of some medicinal plants. Journal of Phytology 3(12): 10-14.
- Han Y, Li LC, Hao WB, Tang M, Wan SQ (2013) Larvicidal activity of Lansiumamide B from the seeds of Clausena lansium against Aedes albopictus (Diptera: Culicidae). Parasitol Res 112(2): 511-516.
- Raja TR, Ganesan P, Gandhi MR, Duraipandiyan V, Paulraj MG (2018) Effect of compound Musizin isolated from Rhamnus wightii Wight and Arn on the immature stages of filarial vector mosquito Culex quinquefasciatus Say (Diptera: Culicidae) and its non-target studies. Biocatal Agric Biotechnol 16: 37-42.
- Prabha J, Anitha PS, Tharayil B (2012) The antimicrobial efficacy of biosynthesized nanostructured ceria. International Journal of Materials Research 111(12).
- Ferdjioui S (2014) Antioxidant and antimicrobial activities of methanolic extracts and essential oil of the plant Mentha Rotundifolia. Doctoral Thesis.
- Hemalatha R, Nivetha P, Mohanapriya C, Sharmila G, Muthukumaran C (1198) Phytochemical composition, GC-MS analysis, in vitro antioxidant and antibacterial potential of clove flower bud (Eugenia caryophyllus) methanolic extract. J Food Sci Technol 53(2): 1189-1198.
- Ghasemi L, Behzad M, Khaleghian A, Abbasi A (2022) Synthesis and characterization of two new mixed-ligand Cu(II) complexes of a tridentate NN'O type Schiff base ligand and N-donor heterocyclic co-ligands: In vitro anticancer Assay, DNA/human leukemia/COVID-19 molecular docking studies, and pharmacophore modeling. Appl Organomet Chem 36(5): e6639.
- Morris GM, Ruth H, Lindstrom W, Sanner MF, Belew RK, et al. (2009) Software news and updates autoDock4 and autoDockTools4: Automated docking with selective receptor flexibility. J Comput Chem 30(16): 2785-2791.
- Dodke P, Pansare T (2017) Ayurvedic and modern aspect of Terminalia chebula Retz. Haritaki An Overview. International Journal of Ayurvedic and Herbal Medicine 7: 2508-2517.
- Khan KH (2009) Terminalia Chebula reduces the oxidative stress induced by Salmonella typhimurium in mice and may reduce the risk of getting typhoid. Adv Biol Res (Rennes) 3: 1-08.
- Mammen D, Sandhya B, Ramesh S (2012) An investigation to variation in constituents in the fruits of Terminalia chebula Retz at different maturity stages. Int J Pharm Bio Sci 3(1): 416-419.
- Contini S (2017) Typhoid intestinal perforation in developing countries: Still unavoidable deaths? World J Gastroenterol 23(11): 1925-1931.
- Naher S, Ferdous B, Datta T, Rashid F, Tasnim N, et al. (2013) Ayurvedic influences in folk medicine: A case study of a folk medicinal practitioner of Jhalokathi in Barisal district, Bangladesh. Journal of Sustainable Agriculture 7: 295-305.
© 2023, Karishma Kundu. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






