- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Sublingual Immunotherapy Treatment for Asthmatic-Allergic Rhinitis Patients Induced by House Dust Mice
Noureen*
Master’s degrees in Public Health and Political Science, Quiad-e-Azam University, Pakistan
*Corresponding author: Noureen, Master’s degrees in Public Health and Political Science, Quaid-e-Azam University, Islamabad, Pakistan
Submission: August 10, 2022; Published: September 22, 2022

ISSN 2578-0190 Volume6 issues2
Abstract
Background: House Dust Mite (HDM) allergy a much prevalent IgE-mediated indoor allergy is a considerable healthcare burden associated with the allergic rhinitis and bronchial asthma. Different studies reported that HDM is a most probable inhalant allergen cause to the allergic rhinitis and bronchial asthma. This condition may lead to chronic or more severe allergic rhinitis with asthma if the patient exposed to the HDM for a long time. Most promising treatment of allergen immunotherapy has been reported not only management but also can prevent asthma with the early treatment. Sublingual Immunotherapy (SLIT) is used to treat allergic rhinitis effectively under the recommendation of the World Allergy Organization. Many studies have been reported that this kind of therapy not only can manage the allergic rhinitis to severe condition, but also prevent to develop into asthma. The objective for this descriptive retrospective study was to evaluate the efficacy of Sublingual Immunotherapy (SLIT) for treating allergic rhinitis patients who has asthma induced by House Dust Mite (HDM) among Punjab, Pakistan population.
Keywords:Asthma; Indoor allergy; Allergic rhinitis; Sublingual immunotherapy
Mini Review
Bronchial Asthma and allergic rhinitis both disorders are of the two different organs as allergic Rhinitis affects the nose and Bronchial Asthma involves the lungs. World Health Organization (WHO) defined Asthma as a disease that occurs due to inflammation of the air passages in the lungs and is characterized by recurring attacks of breathlessness and wheezing [1]. On the other hand, “Global Initiative for Asthma” (GINA) classifies asthma as heterogeneous disease characterized by chronic air ways inflammation, defined by history of respiratory symptoms such as wheeze, shortness of breath, chest tightness and cough vary over time in in intensity together with variable expiratory airflow limitation [2]. Clinical phenotype of allergic asthma is easily recognized with the family history of allergic disease such as allergic rhinitis [3]. Allergic Rhinitis (AR) a much common health problem affects more than one-third population around the globe worldwide involve all ethnic groups [4]. House Dust Mite (HDM) is a common probable inhalant allergen that causes allergic rhinitis [5-8]. HDM long term exposed patients with comorbidity of asthma and allergic rhinitis can transform to chronic severer condition [9-11]. Allergic rhinitis is associated with the onset development and severity of asthma. The condition is frequently under diagnosed in subjects with asthma as over 80% of asthmatics patients have allergic rhinitis. The dual diseases burden of asthma and allergic rhinitis increases as communities are becoming more urbanized like Pakistan. Allergic rhinitis and bronchial asthma impose a considerable socio- economic burden on patients due to medical costs and lost productivity. Since asthma and allergic rhinitis are highly prevalent co-existed disorders in Pakistan, while no data of SLIT treatment is available, thus this study determine the efficacy and safety of SLIT in adult population of Pakistan.
Methodology Ethical Approval
Research approval was taken from ethical committee of hospital research center. All participating patients signed written consent forms before taking part in study. Patients were selected through nonprobability sampling (consecutive technique).
Study design
A total of 114 patients with persistent allergic rhinitis and bronchial asthma induced by HDM were included. All patients had allergic rhinitis symptoms for the whole a year with seasonal bronchial asthma. These patients had received SLIT drops for a total of three year. This retrospective study was conducted at the Allergy and Asthma Centre, Islamabad and Rawalpindi, Pakistan. These health care facilities are catering the needs of allergy and asthma patients since many years. Thus 114 physician diagnosed bronchial asthma were included in this study from October 2018 to October 2019. The patients’ ages were 18 to 70 years old. After taking the approval of ethical committee all consecutive subjects who volunteered for participation in the study were given informed consents. The whole information regarding to the research was given to the participants and written consent were obtained prior the study. Patients who have persistent symptoms of respiratory infections and patients with Chronic Obstructive Pulmonary Disease were excluded from this study. All these patients were undergoing diagnostic protocol with their demographic profile, age, family history, environmental factors of traffic pollution, housing, carpeted rooms and Exposure to pets. These patients were collaborated with E.N.T. department for the sign and symptoms for allergic rhinitis and bronchial asthma. The patients with itching in the nose and palate with blocked or runny nose for long time were considered for nasal smears to check increased eosinophil counts for confirmation of allergic rhinitis. These patients’ severity of the symptoms was classified according to the ARIA guidelines.
The inclusion criteria were patients’ ages >18 years, who had moderate to severe persistent AR with positive nasal provocation test results, with a positive serum HDM-specific IgE level of 3.5 kU/L, according to the Dermatophagoides pteronyssinus and Dermatophagoides farinae. Exclusion criteria was consisted of systematic immunologic disorders such as allergic to food, atopic dermatitis, animal dander; specific IgE level of ≥50 kU/L for other allergic reasons, history of chronic rhino sinusitis, pregnancy; psychological, anaphylactic shock to avoid any affect the outcome assessments; β-blockers and corticosteroids usage 6 months before the study.
Treatment schedule
We used SLIT standard extract drops according to the recommended schedule for bronchial asthma with allergic rhinitis patients. These patients received once daily 1 drop of SLIT (333μg/ mL) for the first month. From second month we added 2 drops of SLIT (1000μg/mL) for a full whole year. Adverse effects of the treatment were recorded during the whole period.
Methodology and Analysis
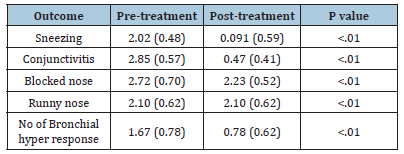
For sample size calculation open epi software was employed. SPSS software version 21 was utilized for statistical analysis. Kolmogorov-Smirnov test was employed for samples distributions. Paired samples t test was used to analyze the quantitative data. Significance level of 5% was set for this study while gender, allergic rhinitis and risk factor exposure was computed for frequency and percentages. Outcome measurements were assessed after one year treatment. For continuous variable of age Mean and Standard deviation was computed. A total of 114 patients having bronchial asthma and allergic rhinitis induced by HDM took part in the study. Patients aged from 18 to 70 years, with mean age of 36.9 years. Among 114 of them, 72 (35.9%) patients were female patients, 42 (20.7%) were males. Out of 114 patients 47 were classified having intermittent asthma, 67 having persistent asthma and further categorized having mild persistent (n=19), moderate persistent (31) and severe persistent asthma (17) according to GINA guidelines. The allergic rhinitis frequency among bronchial asthma patients came out to be 72. Among them 29 were males while 43 were females. These patients were grouped in to intermittent (n=35) and persistent (39) according to ARIA guidelines. The mean duration of the HDM-induced AR and asthma were 9.04 years. Nine patients were moderate persistent with AR, and 83 patients were severe persistent AR. After one year treatment, the SLIT showed promising improvement, compared to before the treatment (P < .01). Majority of the subjects were living in urban area with abundant air pollution. A quarter was exposed to pets and carpeted rooms. Bronchial asthma was more common in our male patients with intermittent. While moderate to severe allergic rhinitis was more common in females. The patients’ adverse events were also recorded during the period of one year treatment. All the patients were asked to regular visit to clinical investigators for outcome measurements for one year treatment, respectively Table 1. All the outcome measurements were assessed before the study and after treatment and no adverse event were observed in this study Table 2.
Table 1:Adverse events during the three years treatment of SLIT.

Table 2:Symptoms noted pretreatment and post treatment.
Discussion
One retrospective study of Italy results showed that SLIT treatment improved AR symptoms with bronchial reactivity significantly and even about 8 years after the treatment [12]. A clinical trial in Korean adult population found that SLIT significantly improved the symptoms in patients with AR induced by HDM [13]. It is found that SLIT can both improve efficacy criteria, measured by rhinitis score and objective criteria, measured by skin reactivity. A Meta-Analysis results found that dust mite SLIT therapy was effective in reducing asthma symptom score [14]. A study from Japan showed efficacy and safety of SLIT in their adults and adolescents’ population [15]. Our study results are also consistent with the previous found that the efficacy of SLIT in patients with AR induced by HDM with acceptable adverse events [15]. SLIT relieve not only the symptoms but also improve patients’ quality of life. Our study results are also consistent with the previous found that the efficacy of SLIT in patients with AR induced by HDM with acceptable adverse events. A study from China on SLIT efficacy in allergic rhinitis patients induced by HDM found improved quality of life in patients [16]. A doubled blind trial form North American HDM-induced allergic rhinitis patients with or without conjunctivitis found well tolerated with improved HDM- induced rhinitis symptoms in patients [17]. Through this institutional based cross-sectional study our finding are consistent in the line with the worldwide studies on SLIT efficacy in allergic rhinitis and bronchial asthma patients. Our study found the promising results that SLIT not only relieve the symptoms of both diseases induced by HDM but can also improve the quality of life in patients. Due to limited time and resources the results are confined for further prospective study with large population of allergic rhinitis and bronchial asthma induced by HDM is required to determine the validity of the results. Moreover, there was no severe adverse event recorded during SLIT treatment period [18-21].
Conclusion
The prevalence is quite high of allergic rhinitis patients with asthma induced by HDM. The results of the present study demonstrated that SLIT treatment may be effective in allergic rhinitis and bronchial asthma patients induced by HDM in Punjab population of Pakistan. In conclusion, the present study not only provided evidence the link between Allergic Rhinitis and Bronchial Asthma induced by HDM but also the efficacy and safety of SLIT for dual disease burden. There is a dire need of the treatment approach to consider the entire airway rather only one part.
Conflict of Interest
Both authors disclose no conflicts of interest.
References
- World Health Organization (WHO) defined.
- (2019) Global initiative for Asthma. Global strategy for Asthma management and prevention.
- Wenzel SE (2012) Asthma phenotypes: the evolution from clinical to molecular approaches. Nat Med 18(5): 716-725.
- Pawankar R, Sánchez-Borges M, Bonini S, Kaliner MA (2013) The burden of allergic diseases. WAO White Book on Allergy: Update, Wisconsin, United States, pp. 27-33.
- Sahin E, Dizdar D, Dinc ME, A A Cirik (2017) Long-term effects of allergen-specific subcutaneous immunotherapy for house dust mite induced allergic rhinitis. J Laryngol Otol 131(11): 997-1001.
- Feng B, Xiang H, Jin H, Haiyong Jin, Jinjian Gao, et al. (2017) Efficacy of sublingual immunotherapy for house dust mite-induced allergic rhinitis: A meta-analysis of randomized controlled trials. Allergy Asthma Immunol Res 9(3): 220-228.
- Kim DH, Kim SW, Kim SW, Jun-Myung Kang (2017) Interleukin-37 relieves allergic inflammation in a house dust mite allergic rhinitis murine model. Iran J Allergy Asthma Immunol 16(5): 404-417.
- Leger D, Bonnefoy B, Pigearias B, Bertrand de La Giclais, Antoine Chartier (2017) Poor sleep is highly associated with house dust mite allergic rhinitis in adults and children. Allergy Asthma Clin Immunol 13: 36.
- Shima K, Koya T, Tsukioka K, Takuro Sakagami, Takashi Hasegawa, et al. (2017) Effects of sublingual immunotherapy in a murine asthma model sensitized by intranasal administration of house dust mite extracts. Allergol Int 66(1): 89-96.
- Fujisawa T, Shimoda T, Masuyama K, Kimihiro Okubo, Kohei H, et al. (2018) Long-term safety of subcutaneous immunotherapy with TO-204 in Japanese patients with house dust mite-induced allergic rhinitis and allergic bronchial asthma: Multicenter, open label clinical trial. Allergol Int 67(3): 347-356.
- Dong X, Huang N, Li W, Lintao Hu, Xiaolong Wang, et al. (2016) Systemic reactions to dust mite subcutaneous immunotherapy: a 3-year follow-up study. Allergy Asthma Immunol Res 8(5): 421-427.
- Marogna M, Bruno M, Massolo A (2007) Long-lasting effects of sublingual immunotherapy for house dust mites in allergic rhinitis with bronchial hyperreactivity: A long-term (13-year) retrospective study in real life. Int Arch Allergy Immunol 142(1): 70-78.
- Kim ST (2015) Outcome of sublingual immunotherapy in patients with allergic rhinitis sensitive to house dust mites. Allergy Asthma Immunol Res 7(2): 99-100.
- Wei Liao, Qi Hu, Lei-Lei Shen, Ying Hu, Hai-feng Tao, et al. (2015) Sublingual immunotherapy for asthmatic children sensitized to house dust mite. Medicine 94(24): e701.
- Okubo K, Masuyama K, Imai T, Toru Imai, Kazuhiro Okamiya, et al. (2017) Efficacy and safety of the SQ house dust mite sublingual immunotherapy tablet in Japanese adults and adolescents with house dust mite-induced allergic rhinitis. J Allergy Clin Immunol 139(6): 1840-1848.
- Han M, Chen Y, Wang M (2018) Sublingual immunotherapy for treating adult patients with allergic rhinitis induced by house dust mite among Chinese Han population a retrospective study. Medicine (Baltimore) Jul 97(30): e11705.
- Hendrik Nolte, David I, Harold S, Jörg Kleine-Tebbe, Gordon L Sussma et al. (2016) Efficacy of house dust mite sublingual immunotherapy tablet in North American adolescents and adults in a randomized, placebo- controlled trial. JACI Global 138(6): 1631-1138.
- Kim SH, Mun SJ, Han DH, Jeong-Whun Kim, Dong-Young Kim, et al. (2015) Three-year follow-up results of sublingual immunotherapy in patients with allergic rhinitis sensitized to house dust mites. Allergy Asthma Immunol Res 7(2): 118-123.
- Chang H, Han DH, Mo JH, Jeong-Whun Kim, Dong-Young Kim, et al. (2009) Early compliance and efficacy of sublingual immunotherapy in patients with allergic rhinitis for house dust mites. Clin Exp Otorhinolaryngol 2(3): 136-140.
- Tonnel AB, Scherpereel A, Douay, Mellin B, Leprince D, et al. (2004) Allergic rhinitis due to house dust mites: Evaluation of the efficacy of specific sublingual immunotherapy. Allergy 59(5): 491-497.
- Novakova SM, Staevska MT, Novakova PI, Yoncheva MD, Bratoycheva MS, et al. (2017) Quality of life improvement after a three- year course of sublingual immunotherapy in patients with house dust mite and grass pollen induced allergic rhinitis results from real-life. Health Qual Life Outcomes 15(1): 189.
© 2022, Noureen. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






