- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Identification of Bacteria Associated With Chicken Omphalitis and Their Antibiotic Profiles
Md Mohibbullah1*, Md Hasibul Hasan2, Md Shofinur Rahman1, Raisa Rafia1, Harunur Rashid2, Mir Rowshan Akter1 and Farzana Afroz1
1Department of Microbiology, Hajee Mohammad Danesh Science & Technology University, Bangladesh
2Faculty of Veterinary, Animal and Biomedical Sciences, Sylhet Agricultural University, Bangladesh
*Corresponding author: Md Mohibbullah, Department of Microbiology, Hajee Mohammad Danesh Science & Technology University, Dinajpur, Bangladesh.
Submission: July 07, 2022; Published: July 15, 2022

ISSN 2578-0190 Volume6 issues1
Abstract
Omphalitis is a non-contagious infection, which affects young poultry’s navel or yolk sac. It’s more likely to happen in an unclean environment, where opportunistic bacterial infection is more common. A study was conducted on the properties of bacteria associated with chicken omphalitis and their antibiotic profile. In this research, we took 55 samples from sick chickens on five separate farms in Dinajpur Sador; Satabganj; Fulbari; and Basherhat; and evaluated them using bacteriological; biochemical; molecular; and antibiotic sensitivity tests. Three bacteria genera (Escherichia coli; Staphylococci aureus; and Salmonella spp.) were isolated from yolk swab samples in omphalitis-infected chicks in this investigation. In both farms, E. coli consumed the maximum percentage of positive cases (94.11%; 80.00%; 83.33%; 71.42%; and 88.88%; respectively); Staphylococcus spp. came in second for the percentage of positive cases (58.82%; 50.00%; 66.66%; 57.14%; and 55.55% respectively); and the percentage of positive cases is the 3rd highest prevalence in Salmonella spp. (32.29 %; 40.00 %; 50.00 %; 42.65 %; and 33.33% respectively).
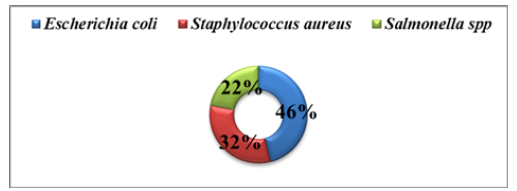
E. coli was the frequent bacteria (46%); subsequently Staphylococcus aureus (32%); and Salmonella spp. (22%). 576 bp DNA fragments were used to identify E. coli, confirming its identification. Isolates E. coli were sensitive to chloramphenicol; gentamycin; levofloxacin; azithromycin; and ciprofloxacin on the other hand resistant to erythromycin; amoxicillin; tetracycline; ampicillin; and Cefixime; according to the drug study. Salmonella spp. was resistant to erythromycin; azithromycin; ampicillin; and tetracycline but sensitive to gentamycin; Cefixime; chloramphenicol; levofloxacin; and ciprofloxacin. Chloramphenicol; levofloxacin; Cefixime; and ciprofloxacin were all effective against Staphylococcus aureus; however, gentamycin; tetracycline; ampicillin; erythromycin; and amoxicillin were not. The outcome of the antibiogram study; has found that all isolates were unaffected against most of the antibiotics; which is not respectable at all. To those antibiotics, which the isolates were susceptible; should be applied in recommended dose for treatment purposes and also need to maintain proper cleaning on environment
Keywords:Antibiotic sensitivity; Bacteria isolation rate; Chicken; Omphalitis; Yolk sac infection
Abbreviations:EMB: Eosine Methylene Blue; SS: Salmonella Shigella; NA: Nutrient Agar; BGA: Brilliant Green Agar; MSA: Manitol Salt Agar; MAC: MacConckey Agar; XLD: Xylose Deoxycholate Agar; TSI: Triple Sugar Iron
Introduction
Omphalitis is a non-contagious disease that affects young poultry’s navel or yolk sac. It’s almost certain in a messy climate where bacteria can spread quickly. In the first fourteen days after hatching, it causes navel discomfort; anorexia; sadness; decreased weight gain; and increased mortality. Omphalitis; also known as yolk sac contamination; most common causes of death in newborn chicks [1]. It arises because of filthy hatchery equipment. Gloomy expressions; hanging heads; and crouching near to the hot source are all symptoms of the affected chicks [2]. The poultry industry in Bangladesh is growing quickly starting around 1980. It undertakes an important part in the destitution lightening and economic improvement of the country. The current rough poultry general population is 300 million (30 cores) including 50 million (5 cores) ducks and 250 million (25 cores) chickens (DLS; October 2012-13); and it incorporates broiler; layer; and duck. There are around 1000 hatcheries facilities in Government and private areas (farm poultry and Livestock survey 2010). The quantity of Day-Old Chicks (DOC) created in Government farms remains at around 125 lakhs to 250 lakhs number each month whereas that of private farms is at around 8 million to 1 core each week [3]. Most frequent causes of death in infants in beginning thereafter brewing is a bacterial infection of the umbilical region [4]. E. coli is the most well-known contaminant in chickenpox; and about 70% of omphalitis-infected chicks have such bacteria in their yolk sac. In contrast, a tiny quantity of E. coli is commonly improved from the normal yolk sac [5].
Open navels contact with the contaminated surfaces is the primary cause of chicken omphalitis. Bacteria can move the patent yolk stem upwards and infect the yolk sac if they meet a contaminated environment before their navel is completely closed. Mixed infections are prevalent; and opportunistic microbes are frequently involved. There is virtually little information on omphalitis in the northern portion of Bangladesh. Even though this problem has become a persistent concern to our chicken sector due to these frequent occurrences at the farmer level; there is no reported study that analyzes the treatments isolation; identification; molecular characterization; and control. The current investigation was done with the isolation and characterization of etiological agents of omphalitis by cultural; biochemical; and molecular approaches with antibiogram profiles; which is a new effort in this region. The goal of this study was to define the bacteria associated with omphalitis in freshly hatched chicks from five hatcheries; as well as to determine which antibiotics are most vulnerable and resistant to omphalitis; which varies from place to location and nation to country.
Materials and Methods
Ethical approval
The methodology of this research was approved by ethical committee of Hajee Mohammad Danesh Science and Technology University [approval number: (HSTU)/IRT/84].
Study design
This study was complemented at the bacteriology research laboratory of the Dept. of Microbiology at Hajee Mohammad Danesh Science and Technology University in Basherhat; Dinajpur; Bangladesh. This study was conducted in January to June 2020. Overall; 55 swab samples were obtained from chicks aged 1 to 7 days from five separate farms. Different bacteriological tests were performed for the characterization of suspected isolates.
Isolation and characterization of bacteria
All swab samples were cultured on NA plates and incubated at 37 °C for 24 hours. Confirmation of microbiological growth some selective media were used for bacterial cultures, such as Staphylococcus agar no. 110; MacConkey agar; Xylose Lysine Deoxycholate agar; EMB agar; Blood agar; SS agar; MSA agar and BGA agar (HI Media; India). All culture plates were sub-cultured and then incubated overnight at 37 °C. By using previously published protocol pure cultures were maintained in a bacteriological laboratory [6]. In this work; several morphological; and biochemical examinations were applied. Gram stain was utilized to assess the morphology and staining properties of microorganisms [6]. Different biochemical tests like Coagulase; catalase; Oxidase; Methyl Red (MR); TSI; Voges-Proskauer (VP); Indole; and Simon’s citrate measures were likewise performed using conventional procedures [7].
Antibiotics susceptibility test
The antimicrobial susceptibility profile of the isolates was assessed using the standard Kirby-Bauer disk diffusion method [8] in accordance with the National Committee for Clinical Laboratory Standards guidelines [9]. Antibiotic sensitivity was investigated using different types of commercial antibiotic discs on a Muller- Hinton agar plate (HI Media). Using sterile forceps; antibiotic disks were administered. Plates were then incubated at 37 °C for 24 hours. After overnight incubation the zone was measured by millimeter scale, according to manufacturer guidelines. 10 commercially available antibiotics was used for the antibiotic sensitivity test (Table 1).
Table 1:Antibiotics with their disc concentration.
Legends: μg: Microgram; SI: Serial; No: Number

Molecular detection
DNA extraction: E. coli genomic DNA isolated since liquid culture 1ml of Nutrient broth overnight growth used in the work. DNA was extracted with a Robotic DNA extractor (Maxwell-16; Source: Promega-USA) as per the maker’s guidance. The genomic DNA was tested for concentration and purity using Nano-Drop Spectrophotometer (ND-2000; Source: Thermo Scientific-USA). The PCR primer: marks gene and PCR cycling summary are showed in Table 2.
Table 2:Oligonucleotide primers structures, mark genes, and cycling situations.

Analysis of PCR products: PCR examination was finished by victimization microorganism by specific primer targeting gene (tetracycline-resistant gene) of E. coli. PCR item was divided by gel electrophoresis utilizing 1.5% of agarose gel marked with ethidium bromide solution and imagined below ultraviolet light by a gel documentation framework.
Data analysis: Data from different farm were entered in excel sheets (Microsoft Excel) and analyzed by using R version 3.6.0. Descriptive analysis summarizing the results. Chi-square test was also used to determine the significance difference in different farm.
Results
Clinical signs
All the chicks handled for the study showed the clinical sign of omphalitis characterized by inflammation of navels, reddish or bluish color of the abdominal muscles around the navel. The postmortem examinations observe an unabsorbed yolk sac in checks.
Identification of bacteria
In this research, 55 samples were collected from infected chickens from five different farms. E. coli; Staphylococcus spp.; and Salmonella spp. were identified from omphalitis infected chicks’ yolk swab samples. Highest prevalence was observed in E. coli 47 (85%); followed by Staphylococcus spp.; 32 (58%) and Salmonella spp. 22 (40%) respectively. Out of 55 samples a total of 101 isolates were isolated from different farms. The results were more or less like [10-12]. E. coli showed the highest % of positive cases in both farm (94.11%; 80.00%; 83.33%; 71.42% and 88.88%) respectively whereas Staphylococcus spp. showed in five farms (58.82%; 50.00%; 66.66%; 57.14%; and 55.55%). The % of positive cases third-ranked in Salmonella spp. in five farms; the percentage is (32.29%; 40.00%; 50.00%; 42.65%; and 33.33% respectively). The significance difference (P<0.05) was observed in nizam and brother poultry farm with highest predominant bacterial isolates (Table 3).
Table 3:Bacteria were detected from a suspected case of omphalitis.

Cultural and morphological finding
The cultural appearances of E. coli; Staphylococcus spp. and Salmonella spp. on different selective media; are observed with different characteristics. E. coli shows green metallic sheen colony on EMB agar; produce whitish colony on XLD agar. Staphylococcus spp. shows Small whitish or yellowish colony on MSA agar while produce small white colony on Staphylococci media no. 110. In XLD agar Salmonella spp. shows red; black center colony. The morphological characteristics of E. coli gives pink colored; rod in shaped; singular arrangement; pair or short chain under microscopy while Staphylococcus spp. shows cluster; cocci shape with purple color. Salmonella spp. appears under microscopy with small rod shape with pink color (Figure 1).
Figure 1:Prevalence of three identify organisms in chicks with clinical signs of omphalitis.
Antibiotic sensitivity test
The isolated Staphylococcus spp. E. coli: and Salmonella spp. were subjected to an antibiotic test to define the sensitivity and resistance pattern against the commonly used antibiotic disc. The results of antibiotic sensitivity tests are presented in Table 4.
Table 4:Antibiogram results of E. coli, Staphylococcus spp. and Salmonella spp.

S: sensitive; I: intermediate; R: resistant
Detection of tetracycline-resistant gene of Escherichia coli
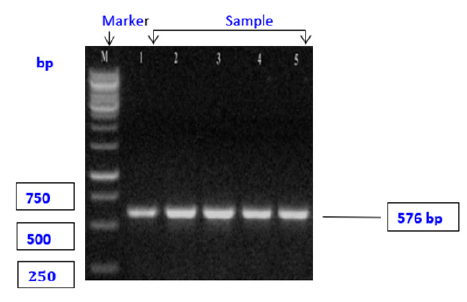
In this study; E. coli in 47 positive samples by cultural; morphological; and biochemical test. Then 5 representative samples were subjected to characterization by PCR; on the PCR technique all E. coli are produce 576 bp band by gel electrophoresis. PCR primers targeting gene (tetracycline-resistant gene) of E. coli amplified 576 bp fragments of DNA confirmed the identity of E. coli. The result of PCR for E. coli is shown in Figure 2.
Figure 2:Profiles of F1 and R1 primers, E. coli showing positive band at 576bp. M: denotes 1kb DNA ladder (Marker). Seg. (bp)= amplified segment.
Discussion
Omphalitis is a non-contagious illness that affects young poultry’s navel or yolk sac. It’s more likely to happen in an unclean environment, where opportunistic bacterial infection is more common. Omphalitis is one of the most serious poultry diseases, causing significant losses. It causes poorer hatchability; greater mortality; and a higher culling rate in afflicted flocks owing to a change in the formation of immunoglobulin proteins followed by bacterial infection; culminating in immunodeficiency [13]. The current study; which involved the isolation of bacterial agents linked to omphalitis in five separate hatcheries in Dinajpur; is the first to indicate the relevance of omphalitis as a source of excessive fatality in chicks at the start of their lives in the research location. E. coli is found in 47 cases: Staphylococcus spp. in 32 and Salmonella spp. in 22 cases (Table 3). This might be related to the increased immunological state of chicks; improved hatchery hygiene conditions; and improved environmental conditions to reduce omphalitis infection in chicks. Several previous studies that showed the common relationship such bacterial isolates with yolk sac infection; agree to findings three primary bacterial isolates in our study from omphalitis infection [2,14,15].
The isolated organism was described using Gram’s staining method and morphological evaluation. Gram staining indicated that the isolated organism was E. coli; which was Gram-negative; rod-shaped; and grouped in single; pair; or short chains. Gram staining revealed Gram-negative; single; or paired short plump rods in Salmonella spp. and Gram-positive cocci-shaped bacteria arranged in the cluster (grape-like) in Staphylococcus spp. with the motility profile of bacteria observed under the microscope after hanging drop slide preparation. Our findings were near closed to [16]. In our current research, morphologically and culturally identified isolates were then using different biochemical tests (MR; VP; Indole; Catalase test; TSI; Coagulase test; etc). This observation also revealed those isolated organisms are E. coli, Salmonella spp. and Staphylococcus spp. with their biochemical feedbacks. The catalase test was positive (presence of bubble) for Staphylococcus spp.; Salmonella spp.; and negative (absence of bubble) for E. coli. Coagulase test was positive (presence of bubble) Staphylococcus spp. and negative (absence of bubble) for E. coli; Salmonella spp. Oxidase test was positive (produce deep purple color) Staphylococcus spp. and negative (no color change) for E. coli; Salmonella spp. Indole tests were also positive (presence of a pink; red-colored circle on the surface of media) for E. coil. Whereas negative (absence of a pink; red-colored circle on the surface of media) for Staphylococcus spp. and Salmonella spp. MR reactions were positive (presence of a red color ring on the surface of media) for E. coil; Staphylococcus spp.; Salmonella spp. VP tests were positive (presence of the red color ring on the surface of the media) for Staphylococcus spp. and negative (absence of a red color ring on the surface of the media) for E. coli and Salmonella spp. MIU tests were positive (diffuse; hazy growth; slightly opaque media) for E. coli and Salmonella spp. (absence of color) for Staphylococcus spp. These findings were also supported by other researchers [2].
In this study, all the suspected isolates obtained from morphological; cultural; and Biochemical studies were subjected to a culture PCR approach. Among three (E. coli; Staphylococcus spp.; and Salmonella spp.) bacterial isolates; only one E. coli; isolates were used for culture PCR for molecular characterization using DNA amplification. In our present study, it was isolated E. coli characterized by using tet A primer sets for the molecular identification and characterization of E. coli amplified at 576 base pairs. All examined E. coli isolated for tetA gene using PCR were totally positive with an incidence of 100% (Figure 2). These findings were supported by [10]. Antibiogram research was performed on the identified isolates in the current study. The findings demonstrated that the field isolates were responsive to a variety of antibiotics utilized in the study to variable degrees. Amoxicillin 50%; ampicillin 67%; Cefixime 67%; Erythromycin 50%; and tetracycline 100% resistance was found in all E. coli isolates. Azithromycin 50%; ampicillin 33%; erythromycin 67%; amoxicillin 17% and tetracycline 67% resistance were found in Salmonella spp. Gentamycin 50%; erythromycin 50%; amoxicillin 33%; tetracycline 67%; azithromycin 33% and ampicillin 67% resistance was found in all Staphylococcus spp. which is related to [2]. Therefore, we strongly suggest using ciprofloxacin; chloramphenicol and levofloxacin against chicken omphalitis.
Conclusion
The researchers wanted to isolate and describe the bacteria that causes yolk sac infection in chicks. E. coli: Salmonella spp. and Staphylococcus spp. were cardinal isolates that causes omphalitis in chicks and prevalence percentages were 85%; followed by 58% and 40% respectively. Our study indicates that ciprofloxacin; chloramphenicol and levofloxacin are more effective treatment against omphalitis. The discovery of antibiotic resistant isolates of E. coli; Salmonella spp. and Staphylococcus spp. against omphalitis in chicks is concerning; resist it could expand to germs affecting human’s livestock. More research is needed in Bangladesh to find policies for the prohibition and dominate of microbial yolk sac infection in chicks.
Acknowledgement
The writers are thankful to the Head of Dept.; research supervisor; Co-supervisor for giving chance to do this type of work and also thankful to the staff of five farms to help me collect the sample for this research The writer also wants to special thanks to IRT.
Acknowledgement
Supervised and designed experiment: MRA and FA.
Performed laboratory work: MM.
Wrote the manuscript: MHH and MHR.
Data analysis: MHH; RR and MSR.
Plagiarism and grammar check: MM; MHH.
All authors read the manuscript before final submission.
References
- Rahman MM, Rahman AZ, Islam MS (2007) Bacterial diseases of poultry prevailing in Bangladesh. Journal of Poultry Science 1(1): 1-6.
- Nasrin S, Islam MA, Khatun M, Akhter L, Sultana S (2012) Characterization of bacteria associated with omphalitis in chicks. Bangladesh Veterinarian 29(2): 63-68.
- Hamid MA, Rahman MA, Ahmed S, Hossain KM (2017) Status of poultry industry in Bangladesh and the role of private sector for its development. Asian Journal of Poultry Science 11(1): 1-3.
- Khalil SA, Einas ES (2012) Aerobic bacteria associated with omphalitis of chicks. Alexandria Journal of Veterinary Sciences 37(1): 69-77.
- Tawab A, Ashraf A, Hofy FI, Nasef SA, Ibrahim OA (2017) Prevalence of eaeA and qacEΔ1 genes in Escherichia coli isolated from omphalitis in baby chicks. Benha veterinary medical journal 32(1): 184-192.
- Merchand IA, Packer RA (1967) Veterinary bacteriology and virology. Veterinary bacteriology and virology.
- Cheesbrough M (2003) Laboratory manual for tropical countries. Volume II, Microbiology, Tropical Health Technology, ELBS, London, UK, pp. 214-220.
- Baur AW, Kirby WM, Sherris JC, Turch M (1966) Antibiotic susceptibility testing by a standardized single disk method. Am J clin pathol 45(4): 493-496.
- Clinical and Laboratory Standards Institute (2017) Performance standards for antimicrobial susceptibility testing. CLSI supplement M100.
- FR ES, AH A, MM H W, AS B, Mwafy A (2019) Antimicrobial resistance and molecular characterization of pathogenic coli isolated from chickens. Journal of Veterinary Medical Research 26(2): 280-292.
- Iqbal M, Shah I, Ali A, Khan M, Jan S (2006) Prevalence and in vitro antibiogram of bacteria associated with omphalitis in chicks. Proteus 26(2): 133-140.
- Nasrin S, Islam MA, Khatun M, Akhter L, Sultana S (2012) Characterization of bacteria associated with omphalitis in chicks. Bangladesh Veterinarian 29(2): 63-6
- Sander JE, Willinghan EM, Wilson JL, Thayer SG (1998) The effect of inoculating Enterococcus faecalis into the yolk sac on chick quality and maternal antibody absorption. Avian diseases 359-363.
- Amer M, ELbayoumi KM, Amin G, Mekky HM, Rabie NS (2017) A study on bacterial contamination of dead in shell chicken embryos and culled one day chicks. Int J Pharm Phytopharmacol Res 7(2): 5-11.
- Abdel-Tawab AA, Nasef SA, Ibrahim OA (2016) Bacteriological and molecular studies on bacteria causing omphalitis in chicks with regard to disinfectant resistance. Global Veterinaria 17(6): 539-45.
- Shahjada Z, Hussain K, Islam MM, Majumder S, Hasan I et al. (2017) Bacteria causing omphalitis in newly hatched chicks from broiler and layer flocks and their antibiotic profiles. Int J Natl Soc Sci 4(2): 73-81.
© 2022,Md Mohibbullah. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






