- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Microbiological Isolates in Patients with Community-Acquired PNEUMONIA, COMPARATIVE STUDY of Three Methods
Luis Alberto Corona Martínez*
Internal Medicine Service, Dr Gustavo Aldereguía Lima University Hospital, Cienfuegos, Cuba
*Corresponding author: Luis Alberto Corona Martínez, Cienfuegos, Cienfuegos province, Cuba
Submission: October 07, 2021; Published: June 21, 2022

ISSN 2578-0190 Volume5 issues5
Abstract
Introduction: Community-acquired pneumonia constitutes a major health problem worldwide.
Objective: to determine the bacterial spectrum in patients hospitalized for pneumonia in a local context.
Method: The results of 208 blood cultures, 69 sputum cultures, and 8 samples of respiratory secretions
and lung tissue obtained postmortem were analyzed. The positivity index was determined for each
method used, as well as the frequency of the isolated germs.
Results: The positivity rate for respiratory secretions (postmortem) was 87.5%, lung tissue 75%,
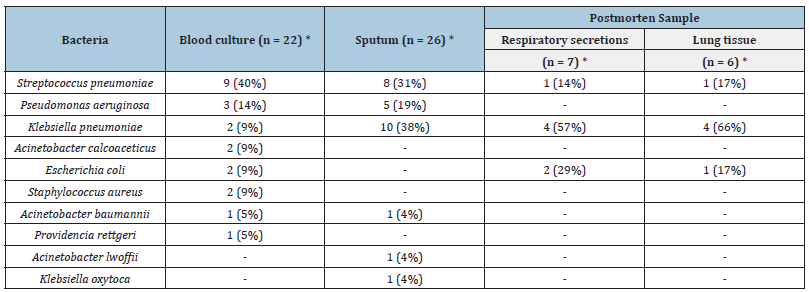
sputum 37.6%, and blood culture 10.6%. The isolates of Klebsiella pneumoniae (20) and Streptococcus
pneumoniae (19) represented 64% of the total isolates.
Conclusion: superiority of sputum culture over blood culture was verified in terms of probability of
microbiological isolation. The importance of Streptococcus pneumoniae and Klebsiella pneumoniae as
causes of community-acquired pneumonia was confirmed.
Keywords:Microbiological isolates; Blood culture; Pneumonia
Introduction
Community Acquired Pneumonia (CAP) is a major health problem worldwide, in Cuba and in the province of Cienfuegos [1-8]. In the specific case of our institution, the Hospital Dr Gustavo Adreima Lima in Cienfuegos, Cuba, the CAP is the first cause of admission to the Internal Medicine service, with figures that exceed a thousand annual admissions. Several guidelines and protocols for the diagnosis and treatment of patients with CAP include carrying out actions aimed at the etiological precision of the infectious process; for this purpose, various means are used, such as blood cultures, cultures of respiratory secretions obtained by expectoration and various serological tests, both in blood and urine [9-14]. Regardless of the usefulness that these complementary tests can provide, the delay in the information they provide and the need for early specific treatment in patients with pneumonia determine that antibiotic therapy in these patients, at the time of hospital admission, continues to be empirical; 2,3,5,10,13 in this sense, the guidelines and protocols are based on the existing epidemiological knowledge about the most frequent causes, both at the universal level and at the local level.
Despite this knowledge, conducting studies aimed at isolating causative agents plays a fundamental role in updating the local microbiological map, with important implications in the updating of therapeutic regimens, fundamentally due to the information related to the development of antimicrobial resistance. In our institution there is a previous experience of attempting to identify the etiology in a series of 52 patients with CAP during the second half of 2012, specifically through the use of blood culture at the time of hospitalization [15]. This new research has aimed, as an objective, to broaden the knowledge of the bacterial spectrum of patients hospitalized for CAP in a local context, for which three different methods of microbiological isolation were compared: blood culture, sputum examination and analysis of samples in deceased patients, both respiratory secretions and lung tissue.
Materials and Methods
Descriptive study, which involved hospitalized patients in the period between April and August 2017 with a coincident admission/discharge diagnosis of CAP based on established clinical, radiological, and pathological criteria (in the case of the deceased) [9-11,16,17]. Three different types of sample units were used: 208 blood cultures, 69 sputum examinations (cultures), and 8 samples of respiratory secretions and lung tissue, respectively, obtained postmortem.
Techniques and Procedures
All the samples were obtained, conserved, transported, and processed following the established technical requirements [18- 22]. The blood culture sample was taken at the time of admission or the day after hospitalization, when the expectoration sample was taken; postmortem samples were taken during autopsies. The results of each sample were reported as: 1) no bacterial growth, 2) contaminated, and 3) isolation. The positivity index (percentage of positive samples out of the total samples) was calculated for each isolation method used, as well as the frequency of the isolated germs, also for each independent method. Because blood culture and sputum examination are common diagnostic tests for pneumonia patients, in addition to being simple procedures that do not entail additional risks for the patient, it was not necessary to request informed consent. The research was approved by the Scientific Council of the institution.
Results
The highest positivity index corresponded to the samples obtained during the necropsy, with practically no differences between the samples of respiratory secretions and those of lung tissue (Table 1). Conversely, the lowest percentage of isolates was found in blood culture. Streptococcus pneumoniae was identified in 40% of the samples obtained by blood culture, thus being the most frequently isolated organism in this method (Table 2). In the sputum examination, this germ reached somewhat lower figures, being surpassed by Klebsiella pneumoniae; in turn, this last bacterium was the most frequent in both types of postmortem samples. In general, a clear predominance of Klebsiella pneumoniae (20) and Streptococcus pneumoniae (19) isolates was found in the samples studied, which represented 64% of the total isolates.
Table 1:Positivity index (in percentage) according to diagnostic method.
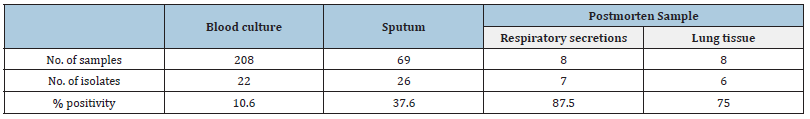
Table 2:Frequency of isolated germs according to diagnostic method.
Discussion
Despite being a highly suggested diagnostic action in the guidelines for the care of patients with CAP, the use of blood culture as a method for etiological diagnosis has raised numerous questions given the generally low frequency of compliance with the objective. In this series, a result very similar to that of the previous investigation is obtained, in which the positivity of the blood culture was 9.6% [15]. In any case, the percentage obtained on this occasion (10.6%) reaffirms the poor diagnostic performance of blood culture at admission in patients with CAP, which has been attributed to reasons such as the previous use of antibiotics and that there is not always bacteremia in these patients, at least at the time of sample collection. Additionally, non-compliance with the technical aspects of the procedure may condition the contamination of the sample,a situation that occurred in this investigation when the isolation of coagulase-negative staphylococcus was recorded in 21 cases, almost the same figure as the productive isolates. However, in the author’s opinion, the simplicity and low cost of blood culture make this test a diagnostic resource of justified indication in patients with severe forms of pneumonia at the time of hospital admission; in patients with a high probability of bacteremia, and in those in whom the more precise etiological diagnosis can be very useful if the clinical evolution is not satisfactory with the initial empirical treatment.
Research aimed at evaluating the diagnostic utility of sputum culture denotes the ancestral interest of physicians in making the expectoration of the pneumonic process beneficial on a diagnostic level [23]. But if the indication for blood culture is controversial, culturing respiratory secretions by collecting expectoration is currently even more controversial. Critical indications towards sputum examination are mainly based on the difficulties for its proper collection (mainly in elderly people, with depressed cough reflex, intense muscle weakness, or with deterioration of the state of consciousness) and the high probabilities of contamination with the flora microbial of the oral cavity [24-26]. Despite the foregoing, this research found a sputum positivity rate much higher than that of the blood culture, which suggests that this test may also be very useful when potentially beneficiary patients are properly selected and, in addition, guarantees are made of technical requirements for proper collection. Other authors point to the full validity of the sputum examination for the etiological diagnosis in the patient with CAP and refer to improvements in the procedures of this complementary diagnostic method [27]. An interesting element of this research was the inclusion of postmortem sampling for the microbiological diagnosis of CAP. Although due to unforeseen technical difficulties the number of samples was very low, both methods, the culture of respiratory secretions and lung tissue, showed not only the highest diagnostic efficiency when compared with blood culture and sputum culture, but also extremely high values of microbiological isolation. Of course, the practical utility of this method in a particular patient is nil; its interest is purely investigative, although later practical implications derive from these investigations. The authors found no references that reflect recent experiences in the use of postmortem samples as part of studies for the etiological diagnosis of CAP.
Regarding the isolated germs, the high frequency of Streptococcus pneumoniae in this series was not surprising. Classically, this bacterium has been recognized as the main cause of community-acquired pneumonia in adults, and in children from two months of age [10,28,29]. In this investigation, a similar frequency of pneumococcal isolation was found in blood and sputum cultures. Among the microorganisms, Klebsiella pneumoniae was isolated in all the methods used in the study, with a frequency that was at the level of that observed for pneumococcus; It was also interesting, the clear predominance of this germ in the sputum samples, unlike that observed in the blood culture. Reviews on the subject indicate that gram-negative bacilli, mainly Klebsiella pneumoniae,are increasingly common as causes of CAP [24]. But studies carried out more than 30 years ago, such as that of Acero and Bermudez, already indicated the parity of isolates between Streptococcus pneumoniae and Klebsiella pneumoniae [23].
Studies also not so recent, where lower positivity rates were obtained than in the present investigation (blood culture 8.8%, sputum 11.9%), had also verified a similar frequency of isolation of Klebsiella pneumoniae and Streptococcus pneumoniae, even with isolation of the first in pleural fluid [30]. Belonging to the enterobacteria, Klebsiella pneumoniae has gained even more relevance today because it is included among the bacteria with the capacity to produce carbapenems [31-33], which relates it to the nefarious problem of antimicrobial resistance and, therefore, with high mortality. In fact, in this study, Klebsiella pneumoniae was the frankly predominant etiological agent in the samples taken from deceased patients, although the possibility of (nosocomial) superinfection in these cases cannot be ignored. As is known, the etiological spectrum of CAP encompasses a wide range of other microorganisms, including Mycoplasma pneumoniae, Chlamydia pneumoniae, Legionella pneumophila, Haemofilus Influenzae, other Enterobacteria, Moraxella catarrhalis, Staphylococcus aureus and anaerobes; some of which are more closely associated with particular clinical situations (gram-negative bacilli in frail elderly; pseudomonas in bronchiectasis) [24,25,34]. In this study, two isolates of Staphylococcus aureus and eight of Pseudomona aeruginosa were made.
As conclusions of the study, the superiority (in this series) of sputum culture over blood culture in terms of probability of microbiological isolation has been verified. Additionally, the recognized predominance of Streptococcus pneumoniae as an etiological agent of CAP has been confirmed, as well as the preponderant role that, in this sense, currently corresponds to Klebsiella pneumoniae; which could have important practical implications, specifically in the initial empirical antibiotic therapy of the patient hospitalized for CAP. The author acknowledge the limitations of the study; these include not having established a positive concordance between the different methods in the same patient, and the non-use of serological methods for triangulating the results of the isolates or the identification of other bacteria of recognized frequency in patients with CAP. (like Mycoplasma pneumoniae, for example). However, in the author’s opinion, both blood and sputum culture continue to be viable options for microbiological diagnosis in patients with community-acquired pneumonia, specifically in selected individuals and under conditions of limited laboratory resources.
Discussion
To Dr. Midiala López Iglesias and Dr. Marleny Array Hernández, for the contribution to the data collection.
References
- Ministry of Public Health (2019) Statistical yearbook of health. National Directorate of Medical Records and statistics of the Ministry of Health, Havana, Cuba.
- Leoni D, Rello J (2017) Severe community-acquired pneumonia: Optimal management. Curr Opin Infect Dis 30(2): 240-247.
- Postma DF, Werkhoven CH, Elden L, Thijsen S, Hoepelman A, et al. (2015) Antibiotic treatment strategies for community acquired pneumonia in adults. N Engl J Med 372(14): 1312-1323.
- GBD 2015 LRI Collaborators (2017) Estimates of the global, regional, and national morbidity, mortality, and aetiologias of lower respiratory tract infections in 195 countries: A systematic analysis for the global burden of disease study 2015. Lancet Infect Dis 17(11): 1133-1161.
- Bender MT, Niederman MS (2018) Treatment guidelines for community-acquired pneumonia. Ann Res Hosp 2: 6.
- Yeon LS, Cha SI, Seo H, Oh S, Choi KJ, et al. (2016) Multimarker Prognostication for hospitalized patients with community-acquired pneumonia. Intern Med 55(8): 887-893.
- Alan M, Grolimund E, Kutz A, Christ Crain M, Thomann R, et al. (2015) Clinical risk scores and blood biomarkers as predictors of long-term outcome in patients with community-acquired pneumonia: A 6-year prospective follow-up study. J Intern Med 278(2): 174-184.
- Viasus D, Del PG, Simonetti AF, García VC, Acosta RJ, et al. (2016) Biomarkers for predicting short-term mortality in community-acquired pneumonia: a systematic review and meta-analysis. J Infect 72(3): 273-282.
- Tsilogianni Z, Grapatsas K, Vasileios L, Zarogoulidis P, Katsikogiannis N, et al. (2017) Community-acquired pneumonia: Current data. Ann Res Hosp 1: 25.
- Noya ME, Moya NL (2017) Diseases of the respiratory system. In: La Habana (Eds.), Acute inflammatory non-tuberculous lung diseases, (5th edn), Part VII, Internal Medicine Topics.
- Torres A, Barberán J, Falguera M, Menéndez R, Molina J, et al. (2013) Multidisciplinary guidelines for the management of community-acquired pneumonia. Med Clin (Barc) 140(5): 223.
- Geijo M, Bermejo E, García A (2014) Diagnostic and therapeutic protocol for community-acquired pneumonia. Medicine 11(52): 3076-3080.
- Girón JA, Pérez S, Girón JA (2018) Diagnosis and empirical treatment of community-acquired pneumonia in special situations: immunocompromised patients without HIV infection and the elderly. Medicine 12(53): 3168-3173.
- Martínez Vernaza S, Mckinley E, Soto MJ, Gualtero S (2018) Community-acquired pneumonia: A narrative review. Univ Med 59 (4).
- González MI, Fragoso MM, Corona ML (2014) Results of performing blood cultures at hospital admission in patients with community-acquired pneumonia. Medisur 12(1).
- Longo DL, Musher DM, Thoner AR, Debakey ME (2014) Community acquired pneumonia. N Engl J Med 371: 1619-1628.
- Monedero MJ, Batalla M, García C, Persiva B, Rabanaque G, et al. (2016) Empirical treatment of adult infections. FMC 23(2): 9-71.
- Cacho JB, Meseguer MA, Oliver A, Puig J (2007) Microbiological diagnosis of bacterial infections of the lower respiratory tract. Procedures in Clinical Microbiology.
- López E (2016) Blood cultures: Nursing procedure and care. Medical Portals Magazine.
- Diagnostic support department. Clinical Pathology Service. Procedures guide, Blood cultures.
- Loza E, Planes RA, Rodríguez CM (2003) Blood cultures. Procedures in Clinical Microbiology.
- Infectious Diseases and Microbiology Clinical Unit (2011) Protocol for the extraction of blood cultures.
- Acero R, Bermudez M (1986) Diagnostic utility of spontaneous sputum and transtracheal aspiration in pulmonary infections. Acta Med Col 11(2): 57-61.
- García Zenón T, Villalobos Silva JA, Trabado López ME (2013) Community pneumonia in the elderly. Evid Med Invest Health 6(1): 12-17.
- Saldías F, Díaz O (2014) Evaluation and management of community-acquired adult pneumonia. Rev Med Clin Condes 25(3): 553-564.
- Reyes IS, Venzant M, García ME, Miro RJ (2011) Update on the diagnosis of community-acquired pneumonia. Medisan 15(7).
- Expósito BM, Drullet PM, Guerra BO, Amigo VM (2016) Improved microbiological diagnosis of lower respiratory infections from expectorated sputum sample. Rev Inf Cient 95(5): 842-850.
- Irastorza I, Landa J, González E (2003) Pneumonia. Etiology and diagnosis. An Pediatr Contin 1(1): 1-8.
- Cemeli CM, Laliena AS, Valiente LJ, Martínez GB, Bustillo AM, et al (2020) Clinical and evolutionary characteristics of community-acquired pneumonia in hospital patients. Rev Pediatr Aten Primaria 22(85): 23-32.
- Anucha A, Linda M (2006) Etiology of community-acquired pneumonia. Case series of the hospital de San José. Repertoire of Medicine and Surgery 26(1): 47-55.
- Paño JR, Serrano VS, Ramos JC (2014) Infections caused by carbapenemase-producing Enterobacteriaceae: Risk factors, clinical features and prognosis. Enferm Infecc Microbiol Clin 32(4): 41-8.
- Valdés ED, Sosa DJ, Sosa DY (2018) Klebsiella pneumoniae, a high priority pathogen for the manufacture of new antibiotics. Matanzas Rev Méd Electrón 40(4): 1271-1273.
- Delfino E, Giacobbe DR, Del BV, Coppo E, Marchese A, et al. (2015) First report of chronic pulmonary infection by KPC-3-producing and colistin-resistant Klebsiella pneumoniae sequence type 258 (ST258) in an adult patient with cystic fibrosis. J Clin Microbiol 53(4): 1442-1444.
- Barberis C, Ledesma M, Álvarez C, Famiglietti A, Almuzara M, et al. (2021) Analysis of the diversity of clinical isolates of Actinomyces/Actinotignum in a university hospital. Rev Argent Microbiol 53(3): 202-209.
© 2022,Luis Alberto Corona Martínez. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






