- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
An Emergence of Multidrug Resistant Nosocomial Pathogen- Acinetobacter Baumannii
Satani S and Ratna Trivedi
Department of Environment Science, India
*Corresponding author: Ratna Trivedi, Department of Environment Science, India
Submission: February 01, 2022; Published: April 21, 2022

ISSN 2578-0190 Volume5 issues5
Abstract
Nosocomial infections have been recognized as one of the most critical problems in hospitalization, particularly in critical care units. As these infections prolong hospitalization, require extensive diagnostics and treatment, leads to high cost. The emergence of multidrug resistant pathogens has become a threat in critically ill, immuno-compromised patients due to the extensive use of antimicrobial. The most common types of nosocomial infections are pneumonia, urinary tract infections, meningitis, wound, soft tissue, surgical site infections and blood stream infections. These infections can be life threatening, capable of making of therapeutic options very difficult and limits the critical care settings. Enterococcus faecium, Staphylococcus aureus, Klebsiella pneumoniae, Acinetobacter baumannii, Pseudomonas aeruginosa, and Enterobacter spp are most common nosocomial pathogens. Among all nosocomial species Multidrug Resistance (MDR) A. baumnaii is most pathogenic microorganism. Here a review on A. baumannii in relation to nosocomial infection is carried out. This review includes risk factors, diagnosis modalities, pathogenesis, MDR properties, mechanism of MDR and treatment of A. baumannii.
Introduction
Acinetobacter baumannii is gram negative, coccobacillus which is ubiquitous in hospital environments. In 1911, Martinis Willem Bergerinck, a Dutch Microbiologist discovered a gram negative, oxidase negative, non-fermentative organism named Micrococcus calcoaceticus from soil on a calcium acetate mineral medium, later on known to be in genus Acinetobacter [1,2]. In 1968 Baumann et al. [3] published comprehensive study on different organisms comprising a single genus, and then the genus Acinetobacter was widely accepted [3]. In late 1970s, Acinetobacter began to be recognized as a nosocomial pathogen [4]. In 1986, Bouvet and Grimont used DNA-DNA hybridization methods to propose 4 new species of Acinetobacter, including Acinetobacter baumannii. According to Wong Acinetobacter baumannii has the ability to persist longer on dry surfaces under nutrient limiting conditions. They have observed that under such conditions the cell wall becomes thicker making it more persistence. This results into rapid transmission in hospitals and other natural environmental [5]. Certain outbreaks studies have revealed that few colonies of strains of Acinetobacter baumannii can remain viable for certain months to years on solid surfaces especially nosocomial surfaces [6]. Acinetobacter baumannii are known as “Iraqibacter” as it was found present in wound infections in soldiers returned from Iraq and Afghanistan. A. baumannii has gained attention in last few years because of its multidrug resistance properties which make its potential nosocomial pathogen [1]. Here a comprehensive review is prepared to summaries recent information and development related to A. baumannii. It has given special emphasized on infections, risk factors, pathogenesis, genetic aspects for virulence and mechanism of antibiotic resistance.
Prevalence of A. Baumannii in various diseases
As described earlier, A. baumannii infections has more prevalence in the patients who are hospitalized due to critical illness, who has serious underlying diseases, who were subjected to invasive and surgical procedures and who were treated with broad spectrum antibiotics for longer duration [7]. It is seen that most of A. baumannii infections involve fluid rich organ systems such as urinary tract, respiratory tract and peritoneal cavity. The most common infections are pneumonia, urinary tract infection, meningitis and trauma. A. baumannii can cause hospital acquired or community acquired pneumonia. In hospitalized acquired pneumonia A. baumannii can be easily isolated from upper respiratory tract but it becomes very difficult to differentiate between upper airway colonization from true pneumonia. Study has shown that A. baumannii is the second most common etiologic agent among all gram-negative bacteria [8]. Only in US surveillance studies have shown that 5 to 10 % of cases from ICUs have A. baumannii infection as patients have longer ICU stay [9]. Study has shown that hospital acquired pneumonia due to A. baumannii in ICU has a frequency of 3-5% with mortality rate of 30-75% [10-16]. Whereas community acquired pneumonia has higher prevalence in Australia and Asia especially in rainy season. Patients with alcohol abuse, smoking, diabetes and COPD are more prone to infection as compared to person with healthy habits. This is characterized by fulminant clinical condition and bloodstream infection. It has a mortality rate of 40% to 60% [10]. The major source of infection for community acquired pneumonia is throat carriage which can be spreaded up to 10% of community residents [11].
Bacteraemia is a condition where blood stream gets infection with bacteria. In majority of cases the origin of bacteraemia is not known. In US Surveillance studies carried out during 1995-2002, it was observed that out of total bacteraemia episodes, around 1.3% of all nosocomial bloodstream infections are because of A. baumannii and it was the 10th most common etiologic agent for bacteraemia. It was seen that in patients who are suffering from bacteraemia and hospitalized in ICU 34%-43% mortality rate whereas patients of outside ICU have only 16% mortality rate. It was also seen that, A. baumannii has initiated more ICU- acquired bloodstream infections as compared to non-ICU ward infections [11,12]. When A. baumannii gets associated with catheter of the patient then it will lead to urinary tract infection. However, the rate of infection is very less having rate of less than 2% of all ICU patients. In case of patients who are outside ICU, infection of A. baumannii is extremely rare [11,13]. Meningitis is a clinical condition where swelling is seen in protective membrane of brain and spinal cord. There are various causes for meningitis including injuries, cancer, certain drug and infection. Recent studies have shown that there is in increase in incidence of nosocomial meningitis due to A. baumannii. It contributes to 10% of total nosocomial infection caused by gram negative bacteria with high mortality rate of around 70% [14]. A. baumannii is also found present in the wound of military population. It was predominant in the wounds of militants returned from Afghanistan or Iraq. Around 32.5% cased were having A. baumannii infection with open tibial fractures. Not only in military population, but also in common population A. baumannii can be seen in wound or skin infection. However, the rate of infection is 2.1% only [7,15,16]. Few reports have also suggested that A. baumannii may cause endocarditis at some extent, especially in the conditions where prosthetic valves are involved. A single case report of bloody diarrhea was reported in an infant due to Acinetobacter haemolyticus strain. Some reports revealed that Acinetobacter spp. may cause keratitis, peritonitis, endopthalmitis related to eye or contact lens surgery [17]. Infection with Multidrug Resistance (MDR) is seen with the patients who were hospitalized for longer period of time especially in ICU. These patients were treated with third generation antibiotics for longer duration which make the microorganisms resistance against them. Studies have shown that the risk factors for MDR strains infection increases many folds in the patient who have undergone surgeries and exposed to infected patients. Treatment of such kind of infection is very difficult and leads to higher mortality rate.
Introduction
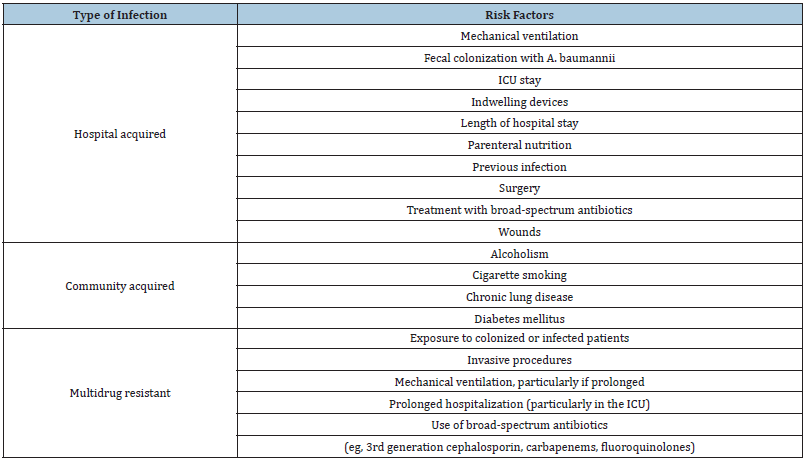
Risk factors for the A. baumannii are varying depending on the type of infection. The below (Table 1) provides complete information about the risk factors associated with type of infection.
Table 1:Risk factors of infections caused by A. baumannii.
Pathogenesis and virulence factors of A. baumannii
In recent years, several virulence factors responsible for the pathogenecity in A. baumannii have been identified using phenotypic and genomic approaches [18]. Major factors include porins, exopolysaccharide, lipopolysaccharides, phospholipase, outer membrane vesicles, protein secretion systems, penicillin binding proteins etc. Porins are made up of protein located on the surface of cell. They are known to play a variety of roles in maintaining cellular structural integrity, bacterial conjugation, antibiotic resistance and pore formation. In A. baumannii, Omp A is one the most abundant porins present in outer membrane. Omp A plays a major role in adherence and invasion. It also induced apoptosis by releasing cytochrome c and complement resistance [18,19]. Omp A also regulates biogenesis of outer membrane vesicles and facilitates surface motility and biofilm formation [20,21]. Other porins namely Omp33-36 are responsible for cytotoxicity action through water passage channel. One study it was also shown that Omp33-36 induces apoptosis in connective tissues and immune cells by modulating autophagy in human cells. Studies have shown that deletion of the Omp33-36 gene reduces adherence and invasion of human lung epithelial cells [22-25]. In addition to porins, capsular exopolysaccharides and lipoproteins can also contribute to pathogenecity. Study has shown that the K locus has a conserved gene cluster in the patients having A. baumannii which determine the production of capsular polysaccharides responsible for pathogenicity [26]. A study has revealed that antibiotics induce hyper-production of capsular polysaccharides, which increases resistance against host complement and increase its virulence [27]. In a study carried out by Liou et al. [28] has demonstrated that the presence of BFMS as virulence factor plays an important role in biofilm formation, adherence to eukaryotic cells, and resistance to human serum [28]. Lipo polysachharides, are biomolecules made up of lipid A moiety, an oligosaccharide core and O antigen. They are immuno-reactive molecules which plays vital role in virulence and induce production of TNF and IL-8 from macrophages [29]. Phospholipases are the enzymes responsible for phospholipids metabolism. These might be virulence factors in many bacteria. They are broadly classified into three classes such as A, C and D. Among the three, PL-C and PL-D act as virulence factors in A. baumannii. In a study carried out by Camarena, they have shown that inactivation of two genes associate with phopholiase namely A1S_0043 and A1S_2055 lead reduction in cytotoxicity [29]. Sthal in their study has revealed that the virulence in A. baumannii strain ATCC 19606 is mediated by concreted action of three PLDs [30]. In addition to LPS and porins, Outer Membrane Vesicles (OMVs) also play vital role on pathogenesis.
Table 2:Functions of virulence determinants possessed by A. baumannii.
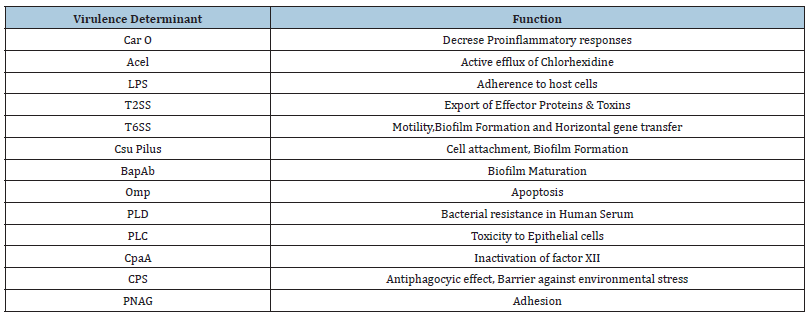
These OMVs are composed of LPS, periplasmic proteins, phospholipids, DNA/RNA. These molecules are secreted by outer membranes of gram-negative bacteria having 20-200nm diameters. OMVs facilitate the pathogen to interact with the host cell without close contact. A. baumannii possess several OMVs belong to phospholipases and proteases, which are responsible for virulence [31,32]. Previous studies have revealed that the presence of outer membrane vesicles can act as an acellular vaccine in A. baumannii strains [33]. A. baumannii also known to have type II protein secretion system (T2SS) which is a multi-protein complex having similar structure as type IV pilli system [34]. T2SS is composed of pseudopilus, ATPase, inner-membrane assembly and outer-membrane dodameric complex. The whole assembly is responsible for export of effector proteins [34,35]. These proteins are responsible for virulence factor to invade into the host cells. T6SS is another protein system which was first identified in Pseudomonas aeuroginosa and Vibrio cholera. T6SS is composed of accessory factors and structural proteins and has a spike like structure to penetrate the target cell [35]. Type 5 secretory system of A. baumannii was found to mediate the biofilm formation and adherence to extracellular matrix which enhances the virulence efficiency [36]. Not only proteins but sometime siderosphores are also secreted from cells. Siderosphores are low molecular weight, iron chelating compound. It is mainly responsible for transportation of iron across the membranes. These siderosphores can modulate the host cellular pathways and add on virulence. Studies have confirmed that A. baumannii contains siderosphores as well as acinetobactin. Acinetobactin is a special class of siderosphore having catecholate and hydroxamate function groups, which can lead to impaired biosynthesis leading cell damage. In addition to all the above factors some other factors were also known to contribute to virulence characteristic. These factors as penicillin binding protein (PBP7/8), CipA, Sur A1, Tuf ect [36-51]. Below table summarize all major virulence determinant possessed by A. baumannii (Table 2).
Mechanism of antibiotic resistan
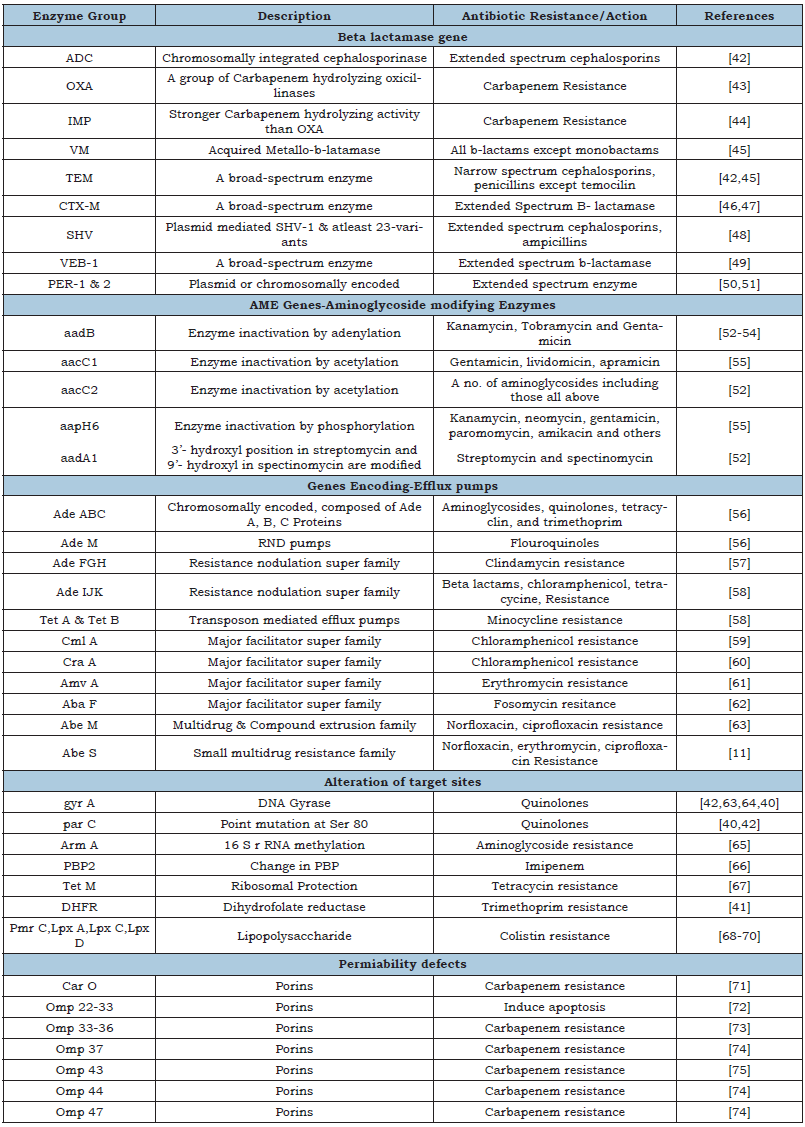
Antibiotic resistance properties of A. baumannii make it more fatal pathogen as compared to other common pathogens. These include various enzymes, structural modifications and genetic alterations. Among all the enzymes β-lactamase is a key enzyme which provides protection against penicillin’s, cephamycin’s, cephalosporins, and carbapenems. All these antibiotics are commonly known as β-lactum antibiotics as they contain β-lactum ring having four carbons. β-lactamase hydrolyse this structure and destroy all the antibiotics resulted into antibiotic resistance. Even it was observed that certain species of A. baumannii are able to resist carbapenems, a strong antibiotic used for MDR microorganisms. Beta metallo-beta lactamases and a class of D OXA of A. baumannii are known to destroy the drug and provide the resistance. A. baumannii was also known to alter the structure of porin and other proteins. Because of this modification antibiotics cannot enter into the cells and not able to destroy the pathogen. Study has shown that A. baumannii gain resistance against colistin though alteration of membrane permeability only. Efflux pump present in the pathogen is responsible for throwing out the antibiotics out of the cell. There are four type of efflux pumps in A. baumannii which are Major Facililator Superfamily (MFS), Resistance Nodulation Cell Division (RND), Small Multidrug Resistance (SMR) and Multidrug and Toxic Compound Extrusion (MATE). AdeABC gene was found responsible for this efflux activity. In addition to AdeABC gene, two other genes namely gyr A and par C were also found associate with antibiotic resistance. Gyr A is a unit of DNA gyrase and par C is a subunit of topoisomerase IV. Point mutation in these two genes alters the membrane binding efficiency which makes them resistance against quinolones. More details about resistance due to enzymes, gene and efflux are summarised in the Table 3; [1,39-75].
Table 3:Summary of various antibiotic resistance mechanisms in A. baumannii.
Diagnostic modalities of A. baumannii infection
As mentioned earlier there are several sites of infection. Based on the site of infections various dianostic modalities are applied for detection of infection. In case of ventilator associated pneumonia chest radiography, sputum analysis, PCR, oxygen saturation, hemodynamic studies and acute phase reactants are determined. In cathater associated urinary tract infections, urine analysis and culture test are more preferred. In case of blood stream associated infection blood investigation, blood culture and ECG are preferred. In case of surgical site infections blood tests such as CRP, FBC, blood culture and swad culture are more preferred. Not limited to these tests, many other tests are also suggestive of A. bauminnii infection depending on the site of infection [10,13,17,18,76].
Treatment
After the discovery of antibiotics in the early 19thcentury, treatment of most of the infectious diseases was carried out using antibiotic. Excessive use of these antibiotics results into genesis of antibiotic resistance microorganisms which are not easily controlled by regular antibiotics. In case of Acinetobacter infections initially ampicillin, gentamicin and nalidixic acid, either as mono or combination were highly used, and it was effective also at that time. But after few years, strains of Acinetobacter became resistance against these antibiotics. Hence, infection disease society of America has considered this specie as red alert pathogen [11]. This high resistance capacity against broad spectrum antibiotic leads to discover the new molecules and re-evaluation of old molecules for efficacy. This is being achieved by applying knowledge of pharmacodynamic and pharmacokinetic [77]. Few such antibiotics are now developed which are still effective against A. baumannii infection. Carbapenems like imipenem and meropenem are effective to certain geological location where strains as still sensitive against these molecules. According to a study, latin America has 40% resistance, Europe & North America has 13%-15% resistance, Singapore has 50% resistance, India has 85 % resistance and Pakistan has 62%-100% resistance against these drugs. Hence these drugs are not suitable for these areas. For the other location they can be used. Sulbactam is a beta lactamase inhibitor which has intrinsic bactericidal activity against Acinetobacter, mediated by penicillin binding proteins. Like the carbapenems, susceptibility of these drugs on A. baumannii varies from various geographic locations. In Germany, France and Spain sulbactam is used either as a single agent or as combination with ampicillin or cefoperazone. Studies have shown that combination of sulbactam with penicillin is more effective against A. baumannii as compared to sulbactum alone [78,79]. Study have shown that meningitis, pneumonia, urinary tract infections caused by MDR A. baumannii can be treated with sulbactam with upto 67.5% healing rate [80]. Tigecycine, a derivative of minocycline is highly used for treating community acquired pneumonia, skin infections, bacterimia, UTIs by MDR A. baumannii. Jung has carried out a study where critical patient who was suffering from ventilator associated pneumonia caused by A. baumannii was treated with tigecycline. This drug was proven effective against MDR A. baumnaii [81,82]. In 2005 Sader has reported the very first case of tigecycline resistance. Studies have shown that strains from Isreal have 66% resistance, India has 57.6%, Greece has 74.2% and Saudi Arabia has 56% resistance [83-85]. Colistin, is another broad-spectrum antibiotic which distracts the cell membrane resulting in the loss of integrity causes cell death. It was highly used between 1960s-1970s, but due to neuro and nephrotoxicity it was discontinued [86]. Studies have shown that colistin has 11%-76% of nephrotoxicities [86]. 86 Colistin and/or polymixin E was last alternate for treating A. baumannii infections [87]. In addition to polymixin E other molecules like tigecycine, sulbactam and trimethoprin are also used in combination with colostin [88-91]. Resistance to colistin was first time reported in 1999, in the Czech Republic [88]. Daadmi has reported 1.8% resistance to colistin in Saudi Arebia, Maspi et al. [90] has reported 48.8% resistance in Iran [89,90] and Gupta et al. [91] have reported 53.1% resistance.
Conclusion
Acinetobacter spp. are among one of the most potential nosocomial microorganisms across the globe. It is known to acquire extended resistance to most antimicrobial agents rapidly. As they have potential to survive in hospital environments for a longer duration leading nosocomial outbreaks. In view of this, to control the spread of this organism, suitable safety programmed should be implemented by healthcare facilities. Research should also be focus on development of new antibiotics which have better efficacy and lower resistance. At present the best way to avoid spreading MDR A. baumannii infection is to maintain cleanliness of the hospital and use antibiotic as per prescription. Colistin can be used as a last alternate for treatment of A. baumannii infection. Selection of antibiotic for treatment should be selected wisely keeping the geographical location in mind.
References
- Alsan M, Klompas M (2010) Acinetobacter baumannii: An emerging and important pathogen. J Clin Outcomes Manag 17(8): 363-369.
- Bonomo RA (2010) Pathogenesis of Acinetobacter spp. Case Western Reserve University, USA.
- Baumann P, Doudoroff M, Stanier RYA (1968) Study of the moraxellk group II. Oxidative-negative species (Acinetobacter). J Bacteriol 95(5): 1520-1541.
- Fournier PE (2006) Comparative genomics of multidrug resistance in Acinetobacter baumannii. PLoS Genetics 2(1): 0020007.
- Du X (47) Predictors of mortality in patients infected with carbapenem-resistant Acinetobacter baumannii: A systematic review and meta-analysis. Am J Infect Control 47(9): 1140-1145.
- Yang CH, Su PW, Moi SH, Chuang LY (2019) Biofilm formation in Acinetobacter Baumannii: genotype-phenotype correlation. Molecules 24 (10): 1849.
- Trottier V (2007) Outcomes of Acinetobacter baumannii infection in critically ill burned patients. J Burn Care Res 28(2): 248-254.
- Howard A, Donoghue M, Feeney A, Sleator RD (2012) Acinetobacter baumannii: An emerging opportunistic pathogen. Virulence 3(3): 243-250.
- Gaynes R, Edwards JR (2005) Overview of nosocomial infections caused by Gram-Negative Bacilli. Clin Infect Dis 41(6): 848-854.
- Doughari HJ, Ndakidemi PA, Human IS, Benade S (2011) The ecology, biology and pathogenesis of Acinetobacter spp: An overview. Microbes Environments 26(2): 101-112.
- Peleg AY, Seifert H, Paterson DL (2008) Acinetobacter baumannii: Emergence of a successful pathogen. Clin Microbiol Rev 21(3): 538-582.
- Garnacho MJ (2015) Optimum treatment strategies for carbapenem-resistant Acinetobacter baumannii Expert Rev Anti-Infect Ther 13(6): 769-777.
- Wood GC, Hanes SD, Boucher BA, Croce MA, Fabian TC (2003) Tetracyclines for treating multidrug-resistant Acinetobacter baumannii ventilator-associated pneumonia. Intensive Care Medicine 29(11): 2072-2076.
- Basri R (2015) Burden of bacterial meningitis: A retrospective review on laboratory parameters and factors associated with Death in Meningitis, Kelantan Malaysia. Nagoya J Med Sci 77(1): 59-68.
- Johnson EN, Burns TC, Hayda RA, Hospenthal DR, Murray CK (2007) Infectious complications of open type III tibial fractures among combat casualties. Clinical Infectious Diseases 45(4): 409-415.
- Murray CK (2006) Bacteriology of war wounds at the time of injury. Military Medicine 171(9): 826-829.
- Bergogne BE, Towner KJ (1996) Acinetobacter spp. as nosocomial pathogens: Microbiological, clinical and epidemiological features. Clin Microbiol Rev 9(2): 148-165.
- Antunes LC, Visca P, Towner KJ (2014) Acinetobacter baumannii: evolution of a global pathogen. Pathog Disease 71(3): 292-301.
- Choi CH (2005) Outer membrane protein 38 of Acinetobacter baumannii localizes to the mitochondria and induces apoptosis of epithelial cells. Cell Microbiol 7(8): 1127-1138.
- Gaddy JA (2012) Role of acinetobactin-mediated iron acquisition functions in the interaction of Acinetobacter baumannii strain ATCC 19606T with human lung epithelial cells, galleria mellonella caterpillars, and mice. Infect Immun 80(3): 1015-1024.
- Gaddy JA, Tomaras AP, Actis LA (2009) The Acinetobacter baumannii 19606 OmpA protein plays a role in biofilm formation on abiotic surfaces and in the interaction of this pathogen with eukaryotic cells. Infection and Immunity 77(8): 3150-3160.
- Rumbo C (2014) The Acinetobacter baumannii Omp33-36 porin is a virulence factor that induces apoptosis and modulates autophagy in human cells. Infect Immun 82(11): 4666-4680.
- Smani Y, Dominguez HJ, Pachon J (2013) Association of the outer membrane protein Omp33 with fitness and virulence of Acinetobacter baumannii. J Infect disease 208(10): 1561-1570.
- Smani Y, McConnell MJ, Pachon J (2012) Role of fibronectin in the adhesion of Acinetobacter baumannii to host cells. PloS One 7(4): e33073.
- Smani Y, Pachon J (2013) Loss of the OprD homologue protein in Acinetobacter baumannii: impact on carbapenem susceptibility. Antimicrob Agents Chemother 57(1): 677.
- Kenyon JJ, Hall RM (2013) Variation in the complex carbohydrate biosynthesis loci of Acinetobacter baumannii PloS One 8(4): e62160.
- Geisinger E, Isberg RR (2015) Antibiotic modulation of capsular exopolysaccharide and virulence in Acinetobacter baumannii. PLoS Pathog 11(2): e1004691.
- Liou ML (2014) The sensor kinase BFMS mediates virulence in Acinetobacter baumannii. J Microbiol Immunol Infect 47(4): 275-281.
- Luke NR (2010) Identification and characterization of a glycosyltransferase involved in Acinetobacter baumannii lipopolysaccharide core biosynthesis. Infect Immun 78(5): 2017-2023.
- Stahl J, Bergmann H, Gottig S, Ebersberger I, Averhoff B (2015) Acinetobacter baumannii virulence is mediated by the concerted action of three phospholipases D. PloS One 10(9): e0138360.
- Kwon SO, Gho YS, Lee JC, Kim SI (2009) Proteome analysis of outer membrane vesicles from a clinical Acinetobacter baumannii FEMS Microbiol Let 297(2): 150-156.
- Jin JS (2011) Acinetobacter baumannii secretes cytotoxic outer membrane protein a via outer membrane vesicles. PloS One 6(2): e17022.
- Huang W (2014) Immunization against multidrug-resistant Acinetobacter baumannii effectively protects mice in both pneumonia and sepsis models. PloS One 9(6): e100727.
- Korotkov KV, Sandkvist M, Hol WG (2012) The type II secretion system: biogenesis, molecular architecture and mechanism. Nat Rev Microbiol 10(5): 336-351.
- Repizo GD (2015) Differential role of the T6SS in Acinetobacter baumannii PloS One 10(9): e0138265.
- Bentancor LV, Camacho PA, Bozkurt GC, Pier GB, Maira LT (2012) Identification of ata, a multifunctional trimeric autotransporter of Acinetobacter baumannii. J Bacteriol 194(15): 3950-3960.
- Koenigs A, Zipfel PF, Kraiczy P (2015) Translation elongation factor tuf of Acinetobacter baumannii is a plasminogen-binding protein. PloS One 10(7): e0138398.
- Liu C (2018) Distribution of virulence-associated genes and antimicrobial susceptibility in clinical Acinetobacter baumannii Oncotarget 9(31): 21663-21673.
- Ardebili A, Lari AR, Beheshti M, Lari ER (2015) Association between mutations in gyrA and parC genes of Acinetobacter baumannii clinical isolates and ciprofloxacin resistance. Iranian J Basic Med Sci 18(6): 623-626.
- Drlica K, Zhao X (1997) DNA gyrase, topoisomerase IV, and the 4-quinolones. Microbiol Mol Biol Rev 61(3): 377-392.
- Mak JK, Kim MJ, Pham J, Tapsal J, White PA (2009) Antibiotic resistance determinants in nosocomial strains of multidrug-resistant Acinetobacter baumannii. Journal of Antimicrobial Chemotherapy 63(1): 47-54.
- Hujer KM (2006) Analysis of antibiotic resistance genes in multidrug-resistant Acinetobacter sp. isolates from military and civilian patients treated at the walter reed army medical center. Antimicrob Agents Chemother 50(12): 4114-4123.
- Koh TH, Sng LH, Wang GC, Hsu LY, Zhao Y (2007) IMP-4 and OXA beta-lactamases in Acinetobacter baumannii from Singapore. J Antimicrob Chemother 59(4): 627-632.
- Landman D (2002) Citywide clonal outbreak of multiresistant Acinetobacter baumannii and pseudomonas aeruginosa in Brooklyn, NY. Arch Intern Med 162(13): 1515-1520.
- Docquier JD (2003) On functional and structural heterogeneity of VIM-type metallo-beta-lactamases. J Antimicrobial Chemother 51(2): 257-266.
- Nagano N, Nagano Y, Cordevant C, Shibata N, Arakawa Y (2004) Nosocomial transmission of CTX-M-2 beta-lactamase-producing Acinetobacter baumannii in a neurosurgery ward. J Clin Microbiol 42(9): 3978-3984.
- Potron A, Munoz PL, Nordmann P, Cleary T, Poirel L (2011) Genetic features of CTX-M-15-producing Acinetobacter baumannii from Haiti. Antimicrob Agents Chemother 55(12): 5946-5948.
- LS T, RA (1999) SHV-type beta-lactamases. Curr Pharm Des 5(11): 847-864.
- Naas T (2006) VEB-1 extended-spectrum β-lactamase–producing Acinetobacter baumannii, France. Emerg Infect Diseas 12(8): 1214-1222.
- Jeon BC (2005) Investigation of a nosocomial outbreak of imipenem-resistant Acinetobacter baumannii producing the OXA-23 beta-lactamase in korea. J Clin Microbiol 43(5): 2241-2245.
- Pasteran F (2006) Emergence of PER-2 and VEB-1a in Acinetobacter baumannii strains in the Americas. Antimicrob Agents Chemother 50(9): 3222-3224.
- Shaw KJ, Rather PN, Hare RS, Miller GH (1993) Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev 57(1): 138-163.
- Nemec A, Dolzani L, Brisse S, Broek PV, Dijkshoorn L (2004) Diversity of aminoglycoside-resistance genes and their association with class 1 integrons among strains of pan-European Acinetobacter baumannii J Med Microbiol 53(12): 1233-1240.
- Jones LA, McIver CJ, Kim MJ, Rawlinson WD, White PA (2005) The aadB gene cassette is associated with blaSHV genes in Klebsiella species producing extended-spectrum beta-lactamases. Antimicrob Agents Chemother 49(2): 794-797.
- Rather P (1998) Origins of the aminoglycoside modifying enzymes. Drug Resistance Updates 1(5): 285-291.
- Marchand I, Damier P, Courvalin P, Lambert T (2004) Expression of the RND-type efflux pump AdeABC in Acinetobacter baumannii is regulated by the AdeRS two-component system. Antimicrob Agents Chemother 48(9): 3298-3304.
- Coyne S, Rosenfeld N, Lambert T, Courvalin P, Perichon B (2010) Overexpression of resistance-nodulation-cell division pump AdeFGH confers multidrug resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 54(10): 4389-4393.
- Vilacoba E (2013) Emergence and spread of plasmid-borne tet(B):ISCR2 in minocycline-resistant Acinetobacter baumannii Antimicrob Agents Chemother 57(1): 651-654.
- Coyne S, Courvalin P, Perichon B (2011) Efflux-mediated antibiotic resistance in Acinetobacter spp. Antimicrob Agents Chemother 55(3): 947-953.
- Roca I (2009) CraA, a major facilitator superfamily efflux pump associated with chloramphenicol resistance in Acinetobacter baumannii. Antimicrob Agents Chemotherapy 53(9): 4013-4014.
- Rajamohan G, Srinivasan VB, Gebreyes WA (2010) Molecular and functional characterization of a novel efflux pump, AmvA, mediating antimicrobial and disinfectant resistance in Acinetobacter baumannii. J Antimicrob Chemother 65(9): 1919-1925.
- Sharma A, Sharma R, Bhattacharyya T, Bhando T, Pathania R (2017) Fosfomycin resistance in Acinetobacter baumannii is mediated by efflux through a Major Facilitator Superfamily (MFS) transporter-AbaF. J Antimicrob Chemother 72(1): 68-74.
- Su XZ, Chen J, Mizushima T, Kuroda T, Tsuchiya T (2005) AbeM, an H+-coupled Acinetobacter baumannii multidrug efflux pump belonging to the MATE family of transporters. Antimicrob Agents Chemother 49(10): 4362-4364.
- Barnard FM, Maxwell A (2001) Interaction between DNA gyrase and quinolones: Effects of alanine mutations at GyrA subunit residues Ser (83) and Asp (87). Antimicrob Agents Chemother 45(7): 1994-2000.
- Yu YS, Zhou H, Yang Q, Chen YG, Li L (2007) Widespread occurrence of aminoglycoside resistance due to ArmA methylase in imipenem-resistant Acinetobacter baumannii isolates in China. J Antimicrob Chemother 60(2): 454-455.
- Cayo R (2011) Analysis of genes encoding penicillin-binding proteins in clinical isolates of Acinetobacter baumannii. Antimicrob Agents Chemother 55(12): 5907-5913.
- Ribera A, Ruiz J, Vila J (2003) Presence of the Tet M determinant in a clinical isolate of Acinetobacter baumannii. Antimicrob Agents Chemother 47(7): 2310-2312.
- Adams MD (2009) Resistance to colistin in Acinetobacter baumannii associated with mutations in the PmrAB two-component system. Antimicrob Agents Chemother 53(9): 3628-3634.
- Moffatt JH (2010) Colistin resistance in Acinetobacter baumannii is mediated by complete loss of lipopolysaccharide production. Antimicrob Agents Chemother 54(12): 4971-4977.
- Arroyo L (2011) The pmrCAB operon mediates polymyxin resistance in Acinetobacter baumannii ATCC 17978 and clinical isolates through phosphoethanolamine modification of lipid A. Antimicrob Agents Chemother 55(8): 3743-3751.
- Mussi MA, Relling VM, Limansky AS, Viale AM (2007) CarO, an Acinetobacter baumannii outer membrane protein involved in carbapenem resistance, is essential for L-ornithine uptake. FEBS Letters 581(29): 5573-5578.
- Bou G, Dom, Carmen Q (2000) Characterization of a nosocomial outbreak caused by a Multiresistant Acinetobacter baumannii strain with a carbapenem-hydrolyzing enzyme: high-level carbapenem resistance in baumannii is not due solely to the presence of lactamases. J Clin Microbiol 38(9): 3299-3505.
- Tomas M (2005) Cloning and functional analysis of the gene encoding the 33- to 36-kilodalton outer membrane protein associated with carbapenem resistance in Acinetobacter baumannii. Antimicrob Agents Chemother 49(12): 5172-5175.
- Quale J, Bratu S, Landman D, Heddurshetti R (2003) Molecular epidemiology and mechanisms of carbapenem resistance in Acinetobacter baumannii endemic in New York City. Clin Infect Diseas 37(2): 214-220.
- Dupont M, Pagès JM, Lafitte D, Siroy A, Bollet C (2005) Identification of an OprD homologue in Acinetobacter baumannii. J Proteome Res 4(6): 2386-2390.
- Vijayakumar S, Biswas I, Veeraraghavan B (2019) Accurate identification of clinically important Acinetobacter spp.: an update. Future Science OA 5: 1-18.
- Khan MF, Aziz F (2016) Antibiotic resistance: preparation for post-antibiotic era. EC Microbiology 3: 409-411.
- Urban C (1993) Effect of sulbactam on infections caused by imipenem-resistant Acinetobacter calcoaceticus biotype anitratus. 167(2): 448-452.
- Corbella X (1998) Efficacy of sulbactam alone and in combination with ampicillin in nosocomial infections caused by multiresistant Acinetobacter baumannii. J Antimicrob Chemother 42(6): 793-802.
- Dinc G (2015) Antimicrobial efficacy of doripenem and its combinations with sulbactam, amikacin, colistin, tigecycline in experimental sepsis of carbapenem-resistant Acinetobacter baumannii. New Microbiol 38(1): 67-73.
- Paudel R, Nepal HP (2020) Tigecycline: pharmacological concerns and resistance. International Journal of Basic & Clinical Pharmacology 9(8): 1296.
- Cunha BA, Mcdermott B, Nausheen S (2007) Single daily high-dose tigecycline therapy of a Multidrug-Resistant (MDR) Klebsiella pneumoniae and Enterobacter aerogenes nosocomial urinary tract infection. J Chemother 19(6): 753-754.
- Navon VS, Leavitt A, Carmeli Y (2007) High tigecycline resistance in multidrug-resistant Acinetobacter baumannii. J Antimicrob Chemother 59(4): 772-724.
- Al Agamy MH (2017) First detection of GES-5 carbapenemase producing Acinetobacter baumannii Microbial Drug Resistance 23(5): 556-562.
- Tsioutis C (2016) Clinical epidemiology, treatment and prognostic factors of extensively drug-resistant Acinetobacter baumannii ventilator-associated pneumonia in critically ill patients. Int J Antimicrob Agents 48(5): 492-497.
- Koksal I, Kaya S, Gencalioglu E, Yilmaz G (2016) Evaluation of risk factors for intravenous colistin use-related nephrotoxicity. Oman Med J 31(4): 318-321.
- Ozkan G (2013) How does colistin-induced nephropathy develop, and can it be treated? Antimicrob Agents Chemother 57(8): 3463-3469.
- Hejnar P, Kolár M, Hájek V (1999) Characteristics of Acinetobacter strains (phenotype classification, antibiotic susceptibility and production of beta-lactamases) isolated from haemocultures from patients at the teaching hospital in Olomouc. Acta Univ Palacki Olomuc Fac Med 142: 73-77.
- Baadani AM, Thawadi SI, El Khizzi NA, Omrani AS (2013) Prevalence of colistin and tigecycline resistance in Acinetobacter baumannii clinical isolates from 2 hospitals in Riyadh Region over a 2-year period. Suadi Med J 34(3): 248-253.
- Maspi H, Hosseini HM, Amin M, Fooladi AA (2016) High prevalence of extensively drug-resistant and metallo beta-lactamase-producing clinical Acinetobacter baumannii in Iran. Microb Patholog 98: 155-159.
- Gupta M (2016) Colistin-resistant Acinetobacter baumannii ventilator-associated pneumonia in a tertiary care hospital: an evolving threat. J Hospital Infection 94(1): 72-73.
© 2022,Ratna Trivedi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






