- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Prevailing Scenario of Mucormycosis (Black Fungus) Disease in the Crisis of Covid-19
Yashwant Sompura1, Tansukh Barupal1, Deepa Hada2* and Shweta Bhodiwal3
1Department of Botany, Mohanlal Sukhadia University, India
2Department of Botany, India
3Department of Botany, India
*Corresponding author: Deepa Hada, Department of Botany, Mohanlal Sukhadia University, India
Submission: June 02, 2021; Published: August 11, 2021

ISSN 2578-0190 Volume5 issues3
Abstract
Mucormycosis or black fungal disease is a rare fungal infection worldwide. Recently one in particular fungal disease Mucormycosis (MCM) also known as zygomycosis. India suffering from this fungal infection already in a deep covid19 crisis. The Rajasthan government has declared mucormycosis or black fungus as an epidemic. Cases of black fungus and resultant deaths are also rising in states like Maharashtra, Utter Pradesh, Uttarakhand, Haryana, Gujarat. The state had nearly 100 above cases of black fungus. This black fungal disease caused by a filamentous fungus Mucormycetes which is present naturally in the environment.
Keywords: Mucormycosis; Black fungus; Fungal infection
Introduction
Mortality rate of black fungus disease or mucormycosis is high. The common fungus Rhizopus oryzae is responsible for this fungal infection [1]. The patients of diabetes mellitus, hematopoietic disorder, malignancy is mainly affected by mucormycosis. It is a rare fungal infection. The common species of mucormycetes is Rhizopus and Mucor [2]. Usually, it is not harmful to human but decreased their immunity. The lungs of such type of individuals who are on medications for other health problems which reduces their ability to fight environmental pathogens [3]. These are some difficulties to manage the mucormycosis that are their diagnosis is very difficult and treatment is surgery.
Following are the types of mucormycosis:
a) Rhino-cerebral mucor-mycosis: The rhino-cerebral mucormycosis is a rare infection of sinuses, nasal passages, oral cavity, and brain. The infection result in death.
b) Gastrointestinal mucormycosis: It related to digestive system.
c) Cutaneous mucormycosis: It is common type of mucormycosis which mainly affect the skin. It spreads throughout the skin and treat by surgery.
d) Disseminated mucormycosis: This type of fungal infection spread throughout the body by blood circulation. It affects the brain, liver, spleen.
e) Pulmonary mucormycosis: It is a lungs infection. This type of fungal infection found in patients of cancer, organ or stem cell transplanted [4] (Figure 1).
Figure 1: Mucormycetes fungi.

Classification of mucormycetes:
Phylum: Fungi
Subphylum: Mucormycotina
Family: Mucoraceae
Order: Mucorales
Genus: Rhyzopusor Mucor
Symptoms
These are common symptoms for individuals who are affected by black fungul disease:
a) Fever
b) Vision loss
c) Headaches
d) Stomachache
e) Coughing
f) Shortness of breath
g) Blood vomits
These are the symptoms of Covid-19 Associated Mucormycosis (CAM) patients with covid19 (active/ recovering/postdischarge) Rhino orbito carebral mucormycosis
a) Congestion
b) Nasal discharge
c) Facial pain
d) Swelling
e) Headache
f) Orbital pain
g) Loosening of toothache
h) Double vision pain
Pulmonary mucormycosis
a) Fever
b) Cough
c) Chest pain
d) Pleural effusion
Cutaneous mucormycosis
a) Pain
b) Swelling around blisters
Disseminated mucormycosis
This type of infection spreads throughout the body and causes several organs to infection and may led to shock or death.
Do’s
A. Control hyperglycemia
B. Check blood glucose level
C. Use of steroids judiciously
D. Use antifungal
Transmission
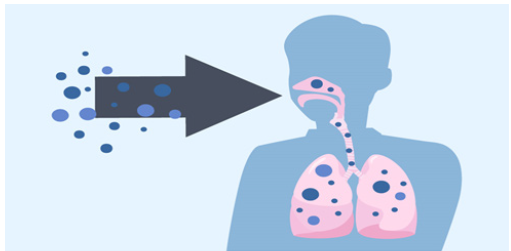
It is transmitted by breathing, inoculation and inhalation of spores present in environment. Such types of spore enter in the lungs and affect lungs. Mucormycosis not transmitted from human to human and human to animal [5] (Figure 2).
Figure 2:Transmission of mucormycosis through breathing.
Diagnosis
If patient has mucormycosis in their lungs or sinuses, then sample of fluid collected from respiratory system and send to a laboratory for diagnosis. Tissue biopsy may perform in the laboratory in which a small sample of affected tissue is analyzed for evidence of mucormycosis under a microscope or in a fungal culture. There is also need imaging tests such as a CT scan of lungs, sinuses, or other parts of body, depending on the location of the suspected infection.
Treatment
Mucormycosis is a serious infection and needs to be treated with prescription antifungal medicine, usually amphotericin B, posaconazole, or isavuconazole. These medicines are given through a vein (amphotericin B, posaconazole, isavuconazole) or by mouth (posaconazole, isavuconazole). Other medicines, including fluconazole, voriconazole, and echinocandins, do not work against fungi that cause mucormycosis. Often, mucormycosis requires surgery to cut away the infected tissue [5-10].
Prevention
a) Use masks when visiting dusty construction sites.
b) Wear shoes, gloves, while handling soil.
c) Maintain personal hygiene.
Conclusion
Mucormycosis is an emerging infection in immunocompromised patients like diabetic patient with neutropenia or neutropenic presenting with rhino-orbitofrontal brain or lung unimproved by appropriate antibiotic therapy. Other locations are less characteristic. Diagnosis is suspected on clinical and radiological features and confirmed by mycological and pathological examination. Treatment consists of amphotericin B combined with surgery. Morbidity and mortality are high due to the invasive nature of the frequent underlying malignancy, hence the importance of early and appropriate management [10-30].
References
- Skiada A, Lass FC, Klimko N, Ibrahim A, Roilides E, et al. (2019) National and Kapodistrian University of Athens, Greece.
- Waldorf AR, Ruderman N, Diamond RD (1984) Specific susceptibility to mucormycosis in murine diabetes and bronchoalveolar macrophage defense against Rhizopus. J Clin Invest 74(1): 150-160.
- Waldorf AR (1989) Pulmonary defense mechanisms against opportunistic fungal pathogens. Immunol Ser 47: 243-271.
- Diamond RD, Haudenschild CC, Erickson NF (1982) Monocyte-mediated damage to Rhizopus oryzae hyphae in vitro. Infect Immun 38(1): 292-297.
- Chamilos G, Lewis RE, Lamaris G, Walsh TJ, Kontoyiannis DP (2008) Zygomycetes hyphae trigger an early, robust proinflammatory response in human polymorphonuclear neutrophils through toll-like receptor 2 induction but display relative resistance to oxidative damage. Antimicrob Agents Chemother 52(2): 722-724.
- Chinn RY, Diamond RD (1982) Generation of chemotactic factors by Rhizopus oryzae in the presence and absence of serum: relationship to hyphal damage mediated by human neutrophils and effects of hyperglycemia and ketoacidosis. Infect Immun 38(3): 1123-1129.
- Lamaris GA, Ben Ami R, Lewis RE, Chamilos G, Samonis G, et al. (2009) Increased virulence of zygomycetes organisms following exposure to voriconazole: a study involving fly and murine models of zygomycosis. J Infect Dis 199(9): 1399-1406.
- Locht M, Boelaert JR, Schneider YJ (1994) Iron uptake from ferrioxamine and from ferrirhizoferrin by germinating spores of Rhizopus microsporus. Biochem Pharmacol 47(10): 1843-1850.
- Boelaert JR, Van Cutsem J, Locht M, Schneider YJ, Crichton RR (1994) Deferoxamine augments growth and pathogenicity of Rhizopus, while hydroxypyridinone chelators have no effect. Kidney Int 45(3): 667-671.
- Maertens J, Demuynck H, Verbeken EK (1999) Mucormycosis in allogeneic bone marrow transplant recipients: Report of five cases and review of the role of iron overload in the pathogenesis. Bone Marrow Transplant 24(3): 307-312.
- Stearman R, Yuan DS, Yamaguchi IY, Klausner RD, Dancis A (1996) A permease-oxidase complex involved in high-affinity iron uptake in yeast. Science 271: 1552-1557.
- Knight SA, Vilaire G, Lesuisse E, Dancis A (2005) Iron acquisition from transferrin by Candida albicans depends on the reductive pathway. Infect Immun 73(9): 5482-5492.
- Jung WH, Sham A, Lian T, Singh A, Kosman DJ, et al. (2008) Iron source preference and regulation of iron uptake in Cryptococcus neoformans. PLoS Pathog 4(2): e45.
- Ibrahim AS, Gebremariam T, Lin L (2010) The high affinity iron permease is a key virulence factor required for Rhizopus oryzae Mol Microbiol 77(3): 587-604.
- Spellberg TJ, Walsh DP, Kontoyiannis JJ, Edwards J, Ibrahim AS (2009) Recent advances in the management of mucormycosis: From bench to bedside. Cli Infect Dis 48(12): 1743-1751.
- Sridhara SR, Paragache G, Panda NK, Chakrabarti A (2005) Mucormycosis in immunocompetent individuals: An increasing trend. J Otolaryngology 34(6): 402-406.
- Mallis A, Mastronikolis SN, Naxakis SS, Papadas AT (2010) Rhinocerebral mucormycosis: An update. Euro Rev Medi & Pharmacol Sci 14(11): 987-992.
- Zilberberg MD, Shorr AF, Huang H (2014) Hospital days, hospitalization costs, and inpatient mortality among patients with mucormycosis: a retrospective analysis of US hospital discharge data. BMC Infec Dis 14: 310.
- Skiada A, Pagano L, Groll A (2011) Zygomycosis in Europe: Analysis of 230 cases accrued by the registry of the European Confederation of Medical Mycology (ECMM) working group on Zygomycosis between 2005 and 2007. Cli Microbiol & Infec 17(12): 1859-1867.
- Roden MM, Zaoutis TE, Buchanan WL (2005) Epidemiology and outcome of zygomycosis: A review of 929 reported cases. Cli Infec Dis 41(5): 634-653.
- Ruping MJ, Heinz WJ, Kindo AJ (2010) Forty-one recent cases of invasive zygomycosis from a global clinical registry. J Antimicro Chemo 65(2): 296-302.
- Rangel Guerra RA, Martınez HR, Saenz C (1996) Rhinocerebral and systemic mucormycosis, clinical experience with 36 cases. J Neuro Sci 143(1-2): 19-30.
- Pagano L, Valentini CG, Posteraroet B (2009) Zygomycosis in Italy: a surveyof FIMUA-ECMM (FederazioneItalianadi Micopatologia Umana ed Animale and European Confederation of MedicalMycology). J Chemo 21(3): 322-329.
- Jeong W, Keighley C, Chen S (2017) The epidemiology, management and outcomes of invasive mucormycosis in the 21st century: a systematic review. ECCMID, p. 1445.
- Gomes MZR, Lewis RE, Kontoyiannis DP (2011) Mucormycosis caused by unusual mucormycetes, non-Rhizopus, -Mucor, and -Lichtheimia species. Clin Microbiol Rev 24: 411-445.
- Ruhnke M, Groll AH, Mayser P (2015) Estimated burden of fungal infections in Germany. Mycoses 58(s5): 22-28.
- Rees JR, Pinner RW, Hajjeh RA, Brandt ME, Reingold AL (1998) The epidemiological features of invasive mycotic infections in the san francisco Bay area, 1992-1993: results of population-based laboratory active surveillance. Clin Infect Dis 27(5): 1138-1147.
- Klimko N, Khostelidi S, Volkova A (2014) Mucormycosis in haematological patients: case report and results of prospective study in Saint Petersburg, Russia. Mycoses 57: 91-96.
- Corzo LDE, Chora HLD, Rodriguez ZP, Walsh TJ (2018) Diabetes mellitus as the major risk factor for mucormycosis in Mexico: epidemiology, diagnosis, and outcomes of reported cases. Med Mycol 56(1): 29-43.
- Lu XL, Najafzadeh MJ, Dolatabadi S (2013) Taxonomy and epidemiology of Mucor irregular is, agent of chronic cutaneous mucormycosis. Persoonia 30: 48-56.
© 2021,Deepa Hada. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






