- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Detecting Total Serum Testosterone by Chemiluminescence Immunoassays, Radioactive Assays, and Liquid-Chromatography Tandem Mass Spectrometry: A Comparative Study
Fenghua Chen1,2,3,4*, Hongxian Zhang5*, Jiatian Song1, Qun Zhong1, Yuan Su1 and Huiyu Xu1,2,3,4
1Center for Reproductive Medicine, China
2Key Laboratory of Assisted Reproduction, China
3National Clinical Research Center for Obstetrics and Gynecology, China
4Beijing Key Laboratory of Reproductive Endocrinology and Assisted Reproductive Technology, China
5Department of Urology, China
*Corresponding author:Fenghua Chen, Center for Reproductive Medicine, China
Submission: February 2, 2021; Published: February 16, 2021

ISSN 2578-0190 Volume4 issues5
Abstract
Aim: LC-MS/MS is still considered the gold standard for detecting steroid hormones in clinic. The aim of this study was to compare the analytical sensitivity of chemiluminescence immunoassays (CLIA) platforms, radioimmunoassay (RIA) platform, and high-performance liquid-chromatography tandem mass spectrometry (LC-MS/MS) platform for detecting total testosterone (tTES) in human samples (males and females).
Method: Sixty-nine healthy male serum samples and 114 female serum samples were collected. The serum testosterone was detected by automatic immunoassay systems of Beckman and SIEMENS, radioimmunoassay, and LC-MS/MS. Pearson’s correlation coefficients were calculated to assess the agreement of other platforms with LC-MS/MS.
Result: When evaluating TES in males, the results detected by SIEMENS2000 had a good correlation with LC-MS/MS (r=0.986), followed by Beckman DXI800, while RIA showed a weak correlation. There was no statistical difference between the results detected by SIEMENS2000 compared to those detected by DXI800 (p=0.87). When evaluating TES in females, DXI800 showed a good correlation with LC-MS/ MS (r=0.8784). SIEMENS2000 had most of the detected values below its low limit, and the SIEMENS2000 results with definite values were not statistically different compared to DXI800 results.
Conclusion: The results of serum tTES detected by two chemiluminescence detection platforms (SIEMENS2000 and DXI800) both had a better correlation with those of LC-MS/MS than RIA. Yet, Siemens 2000 had better consistency with LC-MS/MS in detecting the serums with low tTES value than DXI800.
Keywords: Liquid-chromatography tandem mass spectrometry; Radioimmunoassay; Testosterone; Chemiluminescence immunoassays
Abbreviations: TES: Testosterone; TTES: Total TES
Introduction
Testosterone (TES), a C19 steroid hormone with a molecular weight of 288 Dalton, is a vital androgen in mammals [1]. It is mainly secreted by testicular interstitial cells (Leydig cells) in men and by ovarian and adrenal reticular cells in women [1,2]. TES is the principal androgen hormone essential for the development of secondary sexual characteristics. It promotes protein synthesis, accelerates bone growth, promotes growth and development, and maintains endocrine stability [3]. Many studies suggested that even relatively small changes in steroid hormone levels can cause changes in health status and augment disease risks [4-7]. Thus, it is crucial to detect and measure the total TES (tTES) accurately, even at low concentrations [8]. Accurate measurement of tTES level is crucial for assessment, diagnosis, and monitoring of the treatment of several endocrine disorders. Previous studies have shown that the measurements of plasma or serum tTES levels in males have adequate sensitivity and clinical utility, while they are relatively inaccurate for females and children [9]. The current tTES assays lack accuracy and sensitivity [1,9,10]. Currently, there are no international standards for direct determination of active testosterone in free form. The common immunological methods are based on the reaction of antigen-antibody, such as enzyme linked immunosorbent assay (ELISA), and radioimmunoassay (RIA) for tTES, which have limited sensitivity and accuracy [2,11]. There must be differences between each platform due to the non-unified standards. The aim of this study was to investigate the differences between different chemiluminescence immunoassays (CLIA) platforms and the liquidchromatography-tandemmassspectrometry (LC-MS/MS) platform for determining tTES.
Method
Study population
A total of 183 blood samples (69 samples from male and 114
from female patients) were analyzed in this study. All participants
were in good general health and without serious medical conditions
(acute or chronic). All patients were examined in the Reproductive
Medical Centre, Peking University Third Hospital.
The study was approved by the local Ethics Committee for
Human Studies. All patients gave their written informed consent to
participate in the study.
Instruments and reagents
Testosterone levels were measured using the RIA Testosterone kit (No. IM1087). The CLIA method was performed using UniCel DXI800, an automatic biochemical analyzer from Beckman of USA, and IMMULITE 2000, an automatic biochemical analyzer from SIEMENS of Germany. The reagents were all test kits for the corresponding instruments. LC-MS/MS method was performed using HPLC-MS/MS system, including a high-performance liquid chromatography system (LC-20AD type, Varian, USA), API4000 triple quadrupole mass spectrometer, equipped with electrospray ionization source (ESI) and Analyst 1.4.1 data processing software.
Detection method
CLIA method: A 3mL fresh blood sample were kept at room temperature for 30min. Samples were then centrifuged at 1760g/ min for 10min and analyzed using the American Beckman automatic biochemical analyzer UniCel DXI800 and the German Siemens automatic biochemical analyzer IMMULITE 2000 instrument according to the manufacturers’ instructions, respectively. The remaining serum was detected by the RIA method. Samples were then stored at -20 °C; all the frozen specimens were sent to a highperformance liquid chromatography-tandem mass spectrometry laboratory.
RIA method: The RIA method was conducted as follows: (a) Samples extraction: 200 μL of sample and 2 mL of ethyl ether were mixed and vortex for 1 minute. The vials were kept at<-18°C until the aqueous phase was frozen. The organic phase was then carefully removed, and the ether was evaporated. Finally, the dry ether extracts were re-dissolved in 200 μL of extraction assay buffer. (b) Additions: tubes were coated with antibody (50 μL of calibrator, extracted control or extracted sample, and 300 μL of tracer) and mixes. (c) Incubation: mixture was incubated for 1 hour at 18-25 ℃ with continuous shaking (>400 rpm). Washing and Counting: the content of tubes was carefully aspirated, washed with 2 mL of wash solution, and the bound cpm (B) and total cpm (T) were counted for 1 min.
LC-MS/MS method: The experiment includes pretreatment, detection, and analytical calculations, which were performed as previously described [12]. The parameters for tTES test of different platforms are shown in Table 1. As can be seen from Table 1, the sensitivity of mass spectrometry is the highest of 9.4pg/mL, followed by radioimmunoassay of 0.03ng/mL. The chemiluminescence method of SIEMENS2000 has the lowest sensitivity of 0.15ng/mL.
Table 1:The parameters for testosterone test of different test platforms.
Statistical Analysis
In this study, the correlation coefficients with 95% confidence intervals (CIs) of Beckman, Siemens, RIA, and LC-MS in males and females were calculated. The Z test was used to compare the statistical differences of the three correlation coefficients in males and females, and the statistical differences of the correlation coefficients between males and females in three platforms. SAS 9.4 statistical software was used for statistical analysis, and P < 0.05 was considered statistically significant.
Result
Test results of total testosterone (tTES)
All the 183 samples were detected by RIA and two different chemiluminescence immunoassays platforms (Beckman Unicel DXI800 and Siemens IMMULITE 2000), while 20/69 male samples and 20/114 female samples were measured by LC-MS/MS. Among those, 40 samples (20 males and 20 female samples) were randomly selected. Sixty-three male samples exceeded the highest detection limit of the RIA platform; 3 male samples and 85 female samples were below the lowest detection limit of Siemens IMMULITE2000 platform, and 1 female sample was below the lowest detection limit of Beckman Unicel DXI800 platform. The results of 20 male and 20 female samples detected by LC-MS/MS, RIA, and different CLIA platforms are shown in Figure 1, respectively. Different platforms have different analytical sensitivity. 20 male samples and 20 female samples were detected by LC-MS/MS platform and all have definite values. The LC-MS/MS showed the highest analytical sensitivity with the analytical threshold value of 9.4pg/ml. The sensitivity of the RIA platform was lower than LC-MS/MS with the analytical threshold value of 0.03ng/mL; 63 out of 69 samples were detected by the RIA platform, and the other 6 male samples’ values exceeded the RIA’ reportable range. The sensitivity of Beckman and Siemens platforms was the lowest with the threshold value of 0.1ng/mL and 0.15ng/mL; 1 male sample in the Beckman platform did not have a definite value; 3 male samples and 85 female samples in Siemens 2000 platform did not have definite value because of their values being below the reportable range.
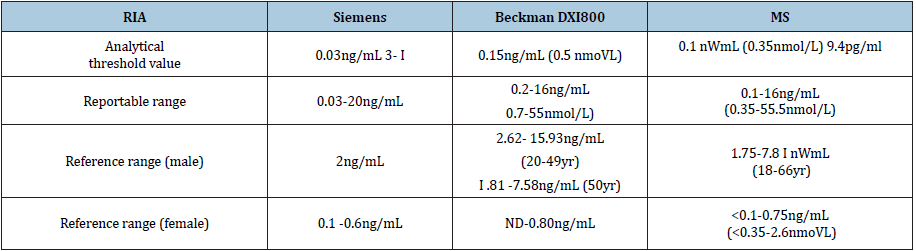
Figure 1:The male testosterone levels measured using four different platforms (RIA, Siemens, Beckman X1800, and MS; n=20/platform).
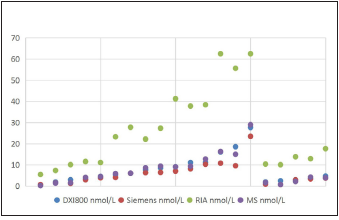
All the 20male samples detected using LC-MS/MS had definite values at other three different platforms, and the results are shown in Figure 1. All the 20 female samples detected using LC-MS/MS had definite values on the RIA platform and DXI800 platform, but 10 out of the 20 serums did not have definite values on the Siemens2000 platform; the results are shown in Figure 2. As shown in Figures 1 & 2, the RIA’ detection values were significantly higher than values detected by the other three platforms; the values detected at the two chemiluminescence platforms are similar to the LCMS/ MS data level (<20nmol/L), and the DXI800’ results are more consistent with the LC-MS/MS’ results than Siemens. Correlation analysis between different platforms and different gender Next, we analyzed the correlation between Beckman, Siemens, RIA, and LCMS/ MS platforms when assessing samples collected from different gender (20 male samples and the 20 female samples) (Figure 3; Table 2). The correlation coefficients between Beckman, Siemens, RIA, and LC-MS/MS for males were r=0.9844, 0.986, 0.9372, while those for females were r=0.8784, 0.8735, and 0.747, respectively. The correlation coefficients of males were higher than females when using the same platform (p<0.05; Table 2).
Figure 2:The female testosterone results in different platforms (RIA, Siemens, Beckman X1800, and MS; n=20/platform).
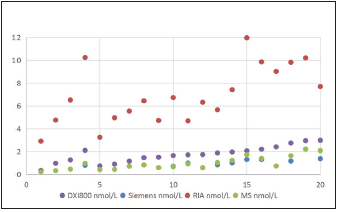
Figure 3:Correlation analysis of Beckman vs. LC-MS.
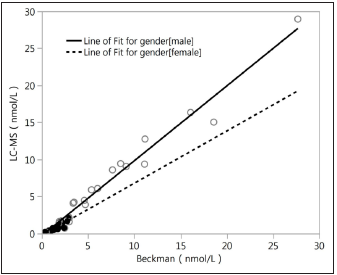
Table 2:Correlation coefficients of three platforms vs. LCMS/ MS, in males and females.
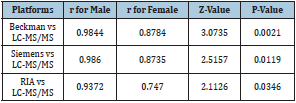
For males, although the results detected by the three platforms were statistically different from those of LC-MS, the results of the three platforms had good consistency with LC-MS (Table 3). Siemens showed the best consistency of 0.9860, followed by Beckman with a consistency of 0.9844; the results of the two platforms were not statistically different (P = 0.8737). When detecting female tTES, a large proportion of cases (85 out of 114) were below the lowest limit when using the Siemens platform, which was rarely observed with Beckman and RIA platforms. The correlation coefficients of Backman and Simense did show a significant difference in the same gender (p=0.8737 in male, p=0.9626 in female). Yet, there was a difference between RIA with the other two platforms in males, and the correlation coefficient of RIA was the lowest.
Table 3:Comparison of r in different platforms.
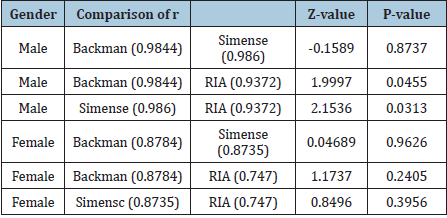
There was no difference between RIA with the other two platforms in females, and the correlation coefficients of the three platforms were all low. The consistency of samples below 0.69nmol/L between the two chemiluminescence immunoassay platforms with LC-MS/MS Based on the above findings, we analyzed the samples with definite values; they were all above the lowest limit of Simense (0.69nmom/L). The samples’ values detected by the RIA platform were obviously higher than LC-MS/MS platform. The correlation coefficient between the two chemiluminescence immunoassay platforms and the LC-MS/MS was higher than that between RIA and LC-MS/MS in males. We further analyzed the samples’ results below 0.69nmol/L when using two chemiluminescence platforms. In order to find which platform was close to the LC-MS/MS, we analyzed the 40 samples’ results detected at LC-MS/MS platform. There were eight results below 0.69nmol/L. These 8 samples’ results were all below 0.69nmol/L at Siemens platform, and only 5 out of the 8 samples’ results were below 0.69 nmol/L at the Beckman platform.
Discussion
Clinical detection of tTES and other steroid hormones are essential for the diagnosis, treatment, and prevention of many diseases. But the analytical performance of individual assays may not meet the needs of all clinical applications [13], especially when dealing with low concentrations, such as tTES in women and estrodiol in men and postmenopausal women [14]. In the present study, we analyzed the analytical sensitivity in detecting tTES using four platforms. The detected values were higher when using the RIA platform than LC-MS/MS platform, both in males and females (Figures 1 & 2). The values detected by platforms Beckman and the Simens2000 revealed good correlation coefficients with those detected by LC-MS/MS platform when the values were higher than 0.69nmol/L; yet, the Simens2000 platform was more consistent with LC-MS/MS than DXI800. Our data suggested that Simens2000 platform represents a better and more accurate choice for tTES detection. The RIA uses radioactive materials in the detection process; thus, it is suitable for clinical use but has danger to human body. The most common tTES detection methods are chemiluminescence and enzyme-linked immunosorbent assay. Yet, their sensitivity may be affected by different work environments, races, etc. [15,16]. For the past ten years, the LC-MS/MS has been considered the gold standard for detecting steroid hormones; this method offers high separation efficiency, high selectivity, and structural specificity for complex samples [17]. Although LC-MS/MS has the most accurate results using gas-phase liquid chromatography, the test is time-consuming and strict on operation conditions and operators. Besides, its equipment is expensive. In this study, we used LC-MS/MS as the standard.
We found statistically different results when using Beckman,
Siemens, RIA, and LC-MS detection platforms (p <0.05), which was
consistent with previous studies [18,19]. In females, the Beckman
platform showed the best consistency with LC-MS (0.8784),
followed by Siemens (0.8735) and RIA (0.747). The consistency of
female samples at all three platforms was lower than that of males.
The consistency was analyzed only by the samples with definite
values; about 70% of female samples could not be analyzed because
of being below the lowest detecting limit of Simens. 0.69nmol/L
was the limitation of the Siemens platform, which had the lowest
sensitivity of all the four platforms. When the results were higher
than 0.69nmol/L, although there was a difference between the
two chemiluminescence platforms with LC-MS/MS, there was no
difference between the Beckman platform and Siemens platform
in the same gender(P>0.05). In the present study, many female
samples did not have definite values in Simens platform because
these values were below the Simens’ low limitation. In order to
compare the two chemiluminescence platforms, we analyzed
the samples’ values which were below 0.69nmo/L in LC-MS/MS
platform.
We found that 8 samples, which were below 0.69nmol/L at
LC-MS/MS platform, all were too low to be detected at Simens
platform, while only 5 out of 8 samples were below 0.69nmol/L at
the Beckman platform. Therefore, the Siemens platform has better
consistency with LC-MS/MS than the Beckman platform when the
values are low. Considering the value of tTE below 0.69nmol/L or
higher than 0.69nmol/L, Simens seems to be the best platform
to substitute LC-MS/MS for detecting low tTES levels. At present,
most countries in the world use the chemiluminescence method
based on antigen-antibody reaction (CLIA) or enzyme-linked
immunosorbent assay to detect testosterone. Still, CLIA has some
shortcomings. Given serum-containing more impurities, the specificity of test results is low. Also, because the standard curve of
CLIA is established according to male androgen level, and the female
androgen level is much lower than in males, testosterone calibration
with low levels is unstable, resulting in low values by each detection
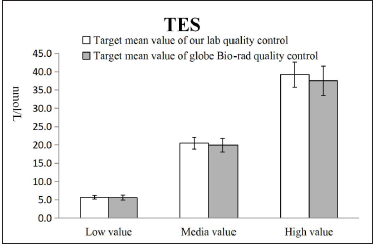
platform with low accuracy. As shown in Figures 4-6, the Quality
Control of the Siemens platform used in our lab was consistent with
the Bio-Rad global Quality Control. We speculated that the lowvalue
assignment to the Quality Control was unstable, which may
be related to unstable calibration, resulting in inaccurate low-value
detection of each detection platform. Because there is no unified
international calibration, the value assigned by each manufacturer
is different. Each machine has good repeat ability; however, the
values of different factory machines are still different. Although
the results of Mass spectrometry are accurate and can be used as
a gold standard, the detection is time-consuming and costly. The
chemiluminescence method is simple, convenient, cheap, and can
be easily used in clinical. Thus, further studies should investigate
the value assigned to the low chemiluminescence method.
Figure 4:Correlation analysis of Siemens vs. LCMS.
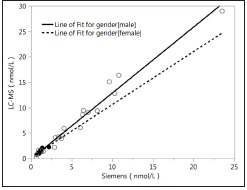
Figure 5:Correlation analysis of RIA vs. LC-MS.
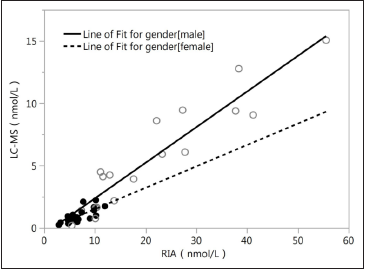
Figure 6:Comparison of quality control of testosterone between our lab with Global Bio-rad.
Acknowledgment
This study was supported by the Major National R&D Projects of China (Grant No. 2017ZX09304012-012); the National Science Fund for Distinguished Young Scholars (Grant No. 81925013, 81701537); the National Key Research and Development Program of China (Grant No. 2018YFC1002104, 2018YFC1002100, 2016YFC1000302); and the Capital Health Research and Development of Special Projects (Grant No. 2018-1-4091).
References
- Wudy SA, Schuler G, Sanchez GA (2018) The art of measuring steroids: Principles and practice of current hormonal steroid analysis. J Steroid Biochem Mol Biol 179: 88-103.
- Dittadi R, Matteucci M, Meneghetti E (2018) Reassessment of the access testosterone chemiluminescence assay and comparison with LC-MS method. J Clin Lab Anal 32(3): e22286.
- Woźniak B, Matraszek ŻI, Sebastian Witek (2017) Development of LC-MS/MS confirmatory method for the determination of testosterone in bovine serum. J Vet Res 61(1): 81-89.
- Würtz AM, Tjonneland A, Christensen J (2012) Serum estrogen and SHBG levels and breast cancer incidence among users and never users of hormone replacement therapy. Cancer Causes Control 23(10): 1711-1720.
- Lin JH, Zhang SM, Rexrode KM (2013) Association between sex hormones and colorectal cancer risk in men and women. Clin Gastroenterol Hepatol 11(4): 419-424.
- Zhang X, Tworoger SS, Eliassen AH (2013) Postmenopausal plasma sex hormone levels and breast cancer risk over 20 years of follow-up. Breast Cancer Res Treat 137(3): 883-892.
- Moskovic DJ, Araujo AB, Lipshultz LI (2013) The 20-year public health impact and direct cost of testosterone deficiency in US men. J Sex Med 10(2): 562-569.
- Vesper HW, Botelho JC, Wang Y (2014) Challenges and improvements in testosterone and estradiol testing. Asian J Androl 16(2): 178-184.
- Taieb J, Mathian B, Millot F (2003) Testosterone measured by 10 immunoassays and by isotope-dilution gas chromatography-mass spectrometry in sera from 116 men, women, and children. Clin Chem 49(8): 1381-1395.
- Büttler RM, Martens F, Ackermans MT (2016) Comparison of eight routine unpublished LC-MS/MS methods for the simultaneous measurement of testosterone and androstenedione in serum. Clin Chim Acta 15: 112-118.
- Moal V, Mathieu E, Reynier P (2007) Low serum testosterone assayed by liquid chromatography-tandem mass spectrometry. Comparison with five immunoassay techniques. Clin Chim Acta 386(1-2): 12-19.
- Xu HY, Jiang H, Feng GS (2020) Establishing the lower limits of total serum testosterone among Chinese proven fertile men who received treatment of assisted reproductive technology. Asian J Androl 22(4): 396-400.
- Van Nuland M, Venekamp N, Wouters WME (2019) LC-MS/MS assay for the quantification of testosterone, dihydrotestosterone, androstenedione, cortisol and prednisone in plasma from castrated prostate cancer patients treated with abiraterone acetate or enzalutamide. J Pharm Biomed Anal 5: 161-168.
- Yuan TF, Le J, Cui Y (2019) An LC-MS/MS analysis for seven sex hormones in serum. J Pharm Biomed Anal 5: 34-40.
- Perheentupa A, Makinen J, Laatikainen T (2013) A cohort effect on serum testosterone levels in Finnish men. Eur J Endocrinol 168(2): 227-233.
- Cawood ML, Field HP, Ford CG (2005) Testosterone measurement by isotope-dilution liquid chromatography-tandem mass spectrometry: validation of a method for routine clinical practice. Clin Chem 51(8): 1472-1479.
- Nisenblat V, Norman RJ (2009) Androgens and polycystic ovary syndrome. Curr Opin Endocrinol Diabetes Obes 16(3): 224-231.
- Herold DA, Fitzgerald RL (2003) Immunoassays for testosterone in women: better than a guess? Clin Chem 49(8): 1250-1251.
- Rosner W, Auchus RJ, Azziz R (2007) Position statement: Utility, limitations, and pitfalls in measuring testosterone: an Endocrine Society position statement. J Clin Endocrinol Metab 92(2): 405-413.
© 2021 Fenghua Chen. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






