- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
An Initial Profile and Virological Response of Sars-Cov-2 Infected Patients Admitted to Infectious Diseases Hospital of Northern India
Reddy Himanshu1, Gupta Shivam1, Khan Farman1, Pandey Saurabh1*, Atam Virendra1, Verma Sudhir1, Kamal Kumar Sawlani1, Shukla Suruchi2, Jain Parul2, Jain Amita2, Vivek Kumar1 and Khan Danish2
1Department of Internal Medicine, India
2Department of Microbiology, India
*Corresponding author: Saurabh Pandey, Department of Internal Medicine, India
Submission: October 20, 2020; Published: December 10, 2020

ISSN 2578-0190 Volume4 issues4
Abstract
Background: SARS-CoV-2 infection has affected the entire world with India being reporting cases since late of February 2020. As India is a tropical country the disease might behave differently and with different outcomes in contrast with temperate ones. Materials and Methods: We enrolled consecutive patients admitted with diagnosis of SARS-CoV-2 and evaluated them by pre-formed proforma to record epidemiology, history, examination and investigation. All cases were diagnosed by RT-PCR for SARS CoV2 from nasopharyngeal sample. Final outcome, hospital stays and viral clearance was estimated.
Result: There were 60 patients with 20% females and 42 (70%) were symptomatic. Mean age was 42.15±5.3 years. 4 were foreign nationals, 3 were infected at religious gathering and 5 (8.3 %) were healthcare. Among symptomatic cases 38 had mild disease with 4 severe cases of pneumonitis of which 2 succumbed to death. Psychological disturbances were seen in 38 (63.3 %) and 50 % patients experienced adverse drug reaction. Minimal response to drug on viral clearance was seen. Multiple organ failure was cause of death in our patients. Loss of smell was observed in 1 case. Mean duration for viral clearance was 9.3±6.8 (2-27) days (range= 4-27 days) and 15 (50%) patients took more than 2 weeks for clearance. Follow up of surviving patients were uneventful.
Conclusion: Asymptomatic and mild disease patients without comorbidities can be excluded from drug therapy and psychological counselling should be a regular support modality. Alternative therapies should be looked into as most of treatments are not effective and viral clearance should not be the end point of treatment.
Keywords: SARS-CoV-2; Northern India; Clinical profile; Viral clearance; Adverse drug effects
Abbreviations: MODS: Multi Organ Dysfunction Syndrome; DIC: Disseminated Intravascular Coagulation; ARDS: Acute Respiratory Distress Syndrome
Introduction
The second pandemic of the century that is the affecting the whole world is SARS-CoV-2 infection. The pathogen has been identified as a novel enveloped RNA betacoronavirus2 that has currently been named severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). It was initially identified at Wuhan city of China and linked to consumption of animal meat at local meat market [1]. It was declared as pandemic on 30th January 2020 by WHO. Currently worldwide cases are 4307287 with mortality of 295101 and affecting 216 countries according to WHO data [2]. India had its first case on 30th January 2020 and since then it is on rise. Our centre was among initial in Uttar Pradesh state of Northern India and we are reporting profile of initial cases admitted to infectious diseases hospital in Northern India with special reference to effect of treatment on viral clearance and adverse drug reaction.
Material and Methods
At the onset of pandemic in India infectious diseases hospital (IDH) under department of medicine at King George Medical University was the initial most centre in the largest state of the country. The patients came to us either by self-reporting for testing, contact tracing, positive test from community screening and elsewhere screening. We enrolled consecutive patients admitted with diagnosis of SARS-CoV-2 and evaluated them by preformed proforma to record epidemiology, history, examination and investigation. All patients were diagnosed based on Indian Council of Medical Research National Institute of Virology criteria and strict screening guidelines levied by the same. All patients were evaluated in terms of resolution of symptoms, viral clearance from nasopharyngeal samples, adverse drug effects and psychological problems faced during stay in hospital along with their outcome. The mandatory test was nasopharyngeal samples for real time polymerase chain reaction (RT-PCR) for SARS-CoV-2. The other test for SARS CoV2 includes- RT-PCR for samples of stool, urine, sputum and blood. Patients were offered treatments in following regimens- Group A: Tab Chloroquine 500mg BD for 5-10 days & tab Oseltamivir 150 mg BD; B: Tab Hydroxychloroquine 400mg BD on day 1 followed by 200mg BD for 5-10 days & tab Azithromycin 500mg BD for 5-10 days. ; C: Tab Hydroxychloroquine 500mg BD for 5-10 days & tab Azithromycin 500mg BD for 5-10 days & Tab Lopinavir/ ritonavir (400/100)mg BD for 7-14 days Routine investigation and chest x ray were also done in all patients after their consent. Written and informed consent was taken from all patients for enrolment in data collection. Ethical clearance for the study was granted by institutional ethical committee.
Result and Discussion
The cumulative results are described in Table 1 (Epidemiologic and clinical characteristics of study population) There were 74 patients who are admitted to our side and 60 patients had outcome at the end of first 3 months. There were 12 females and mean age group was 39.15±5.3 years (range of 2.5 to 78 years). There were 4 foreign nationals (2 each from Bangladesh and Canada). Risk group patient included- 3 from religious gathering, 2 as fruit vendor and 5 infected healthcare staff. 13 patients were diagnosed during contact tracing. 5 patients were screened from hotspot region. 2 of the 3 patients were infected at London and 1 in Turkey. The highest number of individuals infected by single person was 12 in number. 18 (30%) patients were asymptomatic on admission and of them 1 went on to develop fever during stay. Mean oxygen saturation was 92.8±4.3 % and 5 patients had hypoxia at presentation. The clinical picture was characterised by fever with dry cough and variable degree of myalgia, arthralgia, sore throat, and shortness of breath. 1 patient had loss of smell and 2 had diarrhoea. A comparative profile of symptomatic and asymptomatic patients is depicted in Table 2.
Table 1: Epidemiologic and clinical characteristics of study population.
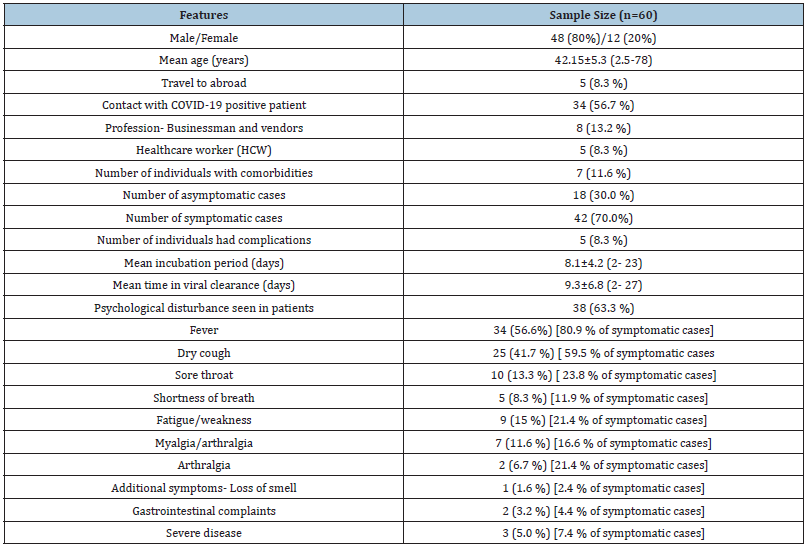
Table 2: Comparative profile among symptomatic and asymptomatic SARS CoV2 infected individuals.
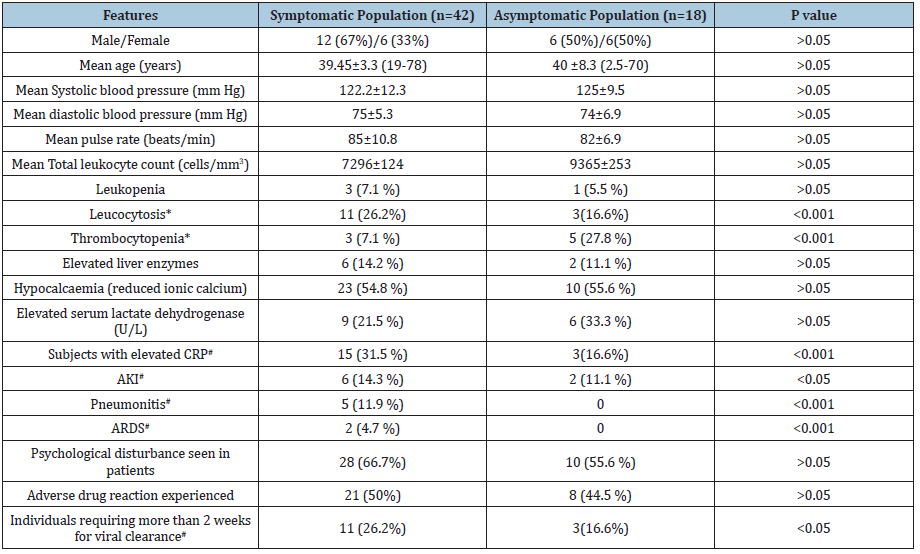
Hb: Haemoglobin; CRP: C Reactive Protein; RBS: Random Blood Sugar; NAD: No Abnormality Detected; HCW: Healthcare Worker; HTN: Hypertension; T2DM: Type 2 Diabetes Mellitus; LDH: Lactate Dehydrogenase.
Among severe diseases 4 had pneumonitis with uncontrolled diabetes and 1 had underlying pre-existing diverticulitis. The notable common laboratory findings included- leucocytosis, thrombocytopenia, hypocalcaemia, elevated serum lactate dehydrogenase and C reactive protein. The latter two along with pneumonitis was seen significantly higher among symptomatic cases. The severe cases had more than one organ system involved. Among the treatment groups as mentioned in method section 5 (8.3%) patients received group A treatment. 42 (70%) received Group B and 3 (5%) received group C. We treated according to availability of literature which was dynamic from the start. The 3 of our severe case received additional lopinavir/ritonavir. 38 (63%) patients experienced one or more psychological features and 30 (50%) experienced adverse drug reaction. During stay 30 (50.0%) patients experienced anxiety, 12 (20%) had mild depression and 3 (5%) experienced impulsive behaviour. 30 (50.0%) experienced medications side effects of which most common were nausea, loss of appetite and abdominal cramps. 1 patient developed hepatitis due to lopinavir/ritonavir and responded after drug withdrawal. Average time for viral clearance was 9.3±6.8 (2-27) days and 15 (25%) patients took more than 2 weeks for clearance. Initial stool samples were done in all patients and were positive for virus. Initial samples of urine and blood were done and came negative. After medications 5 patients symptom improved symptomatically within 3 days of treatment. Average hospital stay was of 17.5 days (range- 3 to 28 days).
This SARS CoV2 infection is a droplet borne infection so has high spread among close contact like MERS and influenza therefore we encountered cases from religious gathering, household contacts and healthcare worker infection [3,4]. The incubation period as shown China CDC was less than 2 weeks with median value of 3 to 7 days [5]. Our data is in accordance with single case with having more than 2 weeks. The common symptoms include-fever, dry cough, dyspnoea, sore throat, malaise, myalgia, fatigue, and diarrhoea. Common complications include- ARDS, MODS, myocarditis and DIC [5,6]. Our patients had similar symptoms in 9 cases and 2 cases had pneumonitis of which one progressed to ARDS with shock leading to death. Study by Bhatraju et al. [7] had 50% mortality in severe disease on invasive ventilation [7]. The factors associated with adverse outcome as estimated by meta-analysis are older patients, male gender, comorbidities, dyspnoea. Non-survivor patients had increased levels of white blood cells, neutrophils, urea, creatinine, creatine kinase, hypersensitive cardiac troponin I, lactate dehydrogenase (LDH), D-dimer, and IL-6. Decreased levels of albumin, lymphocytes, and gas exchange deficit when compared to survivors [8]. Our non-survivor patient had ARDS, AKI, elevated LDH and CRP.
Initial Chinese study showed viral clearance ranged from 4 days to 18 days [5]. The newer study by Liu Y et al. [9] showed viral clearance in mild cases within 10 days of onset of symptoms whereas in severe cases a longer course [9]. In our study mean duration for viral clearance 18.2 days among mild cases which is much higher with a range of 4 to 27 days. This depends upon the duration of sampling for viral detection after exposure and in asymptomatic cases it is not possible to comment on duration of viral clearance. A study by K Xu et al. [10] showed prolonged SARS-CoV-2 RNA shedding was associated with male sex, old age, hypertension, delayed admission to hospital after illness onset, severe illness at admission and mechanical ventilation [10]. The only identifiable risk factor in our study was male sex. At the onset of pandemic in March Chloroquine was the first drug with good in-vitro viral suppression followed by hydroxychloroquine had great expectation in clinical outcome [11,12]. This study by Gao J et al showed improvement in clinical and radiological recovery along with early viral clearance [13]. Thus, initial therapy was in form of chloroquine and oseltamivir was tried but unsuccessful. The adverse drug reaction of the drugs was significant, and the symptoms could be interplay between virus and the drugs.
Since 50 % of our patients had viral clearance after 2 weeks ofstarting treatment and with similar rate of adverse drug reactions the role of present drugs only increased the pill burden and distress of the patient living in long isolation. No cardiac toxicity of hydroxychloroquine with or without azithromycin was seen in our study. The recent review of literature shows no therapeutic option available for its treatment and our cases do not responded to treatment in terms viral clearance [14]. The first case of India was reported on January 30th, 2020 from Kerala state and the first published study from India is by Gupta et al and described 21 patients. 42.9 % patients were asymptomatic. The mean duration for defervescence was 5±1.2 days. Fever with dry cough, sore throat and breathlessness was in 42.9%, 23.8 % and 4.8% respectively and all patients recovered [15]. The second study is by S Bhandari et al. [16] reported 21 patients [16]. Fever with dry cough was seen in around 50% cases and diarrhoea in 38% cases. Almost 50% patient had experienced adverse drug reaction which is very similar to our data. 52.4% patients had lymphopenia whereas in our study it was seen in 10 % and 13.3% had leucocytosis but all were in mild disease patients. Another study by M Saluja et al. [17] and included data of later phase and had similar clinical profile [17]. The symptomatic patients had higher counts, serum LDH, and lymphopenia was seen whereas our patient had higher leucocytosis, CRP and creatinine values. We also experienced hypocalcaemia as a predominant electrolyte abnormality which is not estimated by other studies.
Conclusion
This infection has high transmission rate with most cases as mild disease and poor response to available therapy. In high risk population it can be fatal and morbid. Viral clearance should not be the goal of therapy and rather severe disease should be offered therapy as the drugs side effect profile adversely affects the hospital stay. Prevention is still the best measure available to us.
Acknowledgment
We are thankful to Mr Kamlesh from infectious diseases hospital for proper record maintenance and data collection.
References
2.https://www.who.int/emergencies/diseases/novel-coronavirus-2019
- Tong ZD, Tang A, Li KF (2020) Potential presymptomatic transmission of SARS-CoV-2 Zhejiang Province, China, 2020. China Emerg Infect Dis 26(5): 1052-1054.
- Huang C, Wang Y, Li X (2020) Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. 395(10223): 497-506.
- Cascella M, Rajnik M, Cuomo A (2020) Features, evaluation, and treatment coronavirus (COVID-19).
- Chen N, Zhou M, Dong X (2015) Epidemiological and clinical characteristics of 99 cases of 2019 novel coronavirus pneumonia in Wuhan, China: a descriptive study. Lancet 395(10223): 507-513.
- Liu J, Cao R, Xu M, Wang X, Zhang H, et al. (2020) Hydroxychloroquine, a less toxic derivative of chloroquine, is effective in inhibiting SARS-CoV-2 infection in vitro. Cell Discov 6: 16.
- Gao J, Tian Z, Yang X (2020) Breakthrough: chloroquine phosphate has shown apparent efficacy in treatment of COVID-19 associated pneumonia in clinical studies. Biosci Trends 14(1): 72-73.
- Bhandari S, Bhargava A, Sharma S, Keshwani P, Sharma R (2019) Clinical profile of covid-19 infected patients admitted in a tertiary care hospital in North India. J Assoc Physicians India 68(5): 13-17.
- Saluja M, Pillai D, Jeliya S, Bauddh N, Chandel R (2020) COVID 19- clinical profile, radiological presentation, prognostic predictors, complications and outcome: a perspective from the Indian subcontinent. J Assoc Physicians India 68(7): 13-18.
© 2020 Vahid Bafandegan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






