- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Stealth Adapted Viruses with Genetically Unstable Rhesus Monkey Cellular Sequences A Possible Forerunner of Complex Human Illnesses
W John Martin*
Institute of Progressive Medicine, USA
*Corresponding author: W John Martin, Institute of Progressive Medicine, USA
Submission: August 05, 2020; Published: August 10, 2020

ISSN 2578-0190 Volume4 issues1
Abstract
The production of human virus vaccines in virus-contaminated cultured animal cells provides the opportunity for genetic alterations in the respective vaccine and culture-contaminating viruses. Poliovirus vaccines were previously produced in kidney cell cultures from cytomegalovirus infected rhesus and African green monkeys. Viruses can undergo an immune evasion process termed stealth adaptation. It involves the deletion or mutation of the genes coding for the relatively few virus components that are normally targeted by the cellular immune system. As earlier reported, additional genetic sequences of cellular and bacterial origin can be incorporated into replicating stealth adapted viruses. This article confirms the incorporation of rhesus monkey genome-derived genetic sequences in certain stealth adapted viruses cultured from patients with the chronic fatigue syndrome (CFS). The virus-incorporated cellular-derived sequences differ slightly from the originating cellular sequences reflecting mutational changes and genetic instability. Ongoing mutations are also apparent in the minor differences in the genetic sequences seen in similar PCR products generated from the cultures of the two different CFS patients. Mutated human cellular genome-derived genetic sequences were also detected in the culture from one of the CFS patients. This is consistent with homologous recombination between human sequences and the virus-incorporated monkey cellular sequences. The transmission of genetically unstable, replicating monkey genomic sequences to humans and the potential of further transmission of mutated human genetic sequences between humans, warrants the attention of Public Health officials. The findings also question the continuing use of cultured animal cells to generate virus vaccines for human use.
Keywords: Vaccines; Stealth adapted viruses; Chronic fatigue syndrome; KELEA; Kinetic Energy Limiting Electrostatic Attraction; ACE; Alternative cellular energy
Abbreviations: SV-40: Simian Virus-40; CMV: Cytomegalovirus; SCMV: African green monkey simian cytomegalovirus; CFS: Chronic fatigue syndrome; CPE: Cytopathic effect; PCR: Polymerase chain reaction; KELEA: Kinetic Energy Limiting Electrostatic Attraction; ACE: Alternative cellular energy
Introduction
The inadvertent transmission of animal viruses to humans is a risk of using cultured animal cells to produce human vaccines [1]. Poliovirus vaccines were initially produced in the kidney cells of rhesus monkeys, many of which were infected with simian virus-40 (SV-40) [2-5]. Although formalin was used to inactivate the polioviruses, it was less effective in the inactivation of SV-40. Consequently, many humans were inoculated with live SV-40 virus [3]. A subsequent switch was made in the early 1960s to using African green monkeys, rather than rhesus monkeys. Moreover, the need for formalin inactivation was bypassed by using attenuated polioviruses. Most of the early monkeys used for polio vaccine production were infected with cytomegaloviruses (CMV) [6,7]. It was not surprising, therefore, that in an unpublished joint Food and Drug Administration (FDA) - vaccine manufacturer study conducted in 1972, kidney cell cultures from all eleven tested African green monkeys were shown to be infected with African green monkey simian cytomegalovirus (SCMV) [personal knowledge]. There was no public disclosure of these results seemingly because there had been no reports of acute SCMV illness in vaccine recipients over the prior decade of use of the vaccine. The FDA was similarly reticent to take action when informed in 1995 that molecularly confirmed SCMV-derived atypical viruses were cultured from a patient with the chronic fatigue syndrome (CFS) and from a comatose patient with a 4-year history of a bipolar psychosis [8,9]. The viruses induced a similar, yet distinguishable cytopathic effect (CPE) characterized by the formation of foamy vacuolated cells with considerable cell fusion (syncytia) [10]. The interesting feature of these viruses was their failure to evoke inflammation in the patients from whom the viruses are cultured, or in virus inoculated animals [11]. Based on DNA sequencing data, this was attributed to the deletion or mutation of the genes coding for the relatively few virus components that are normally targeted by the cellular immune system [12]. This immune evasion mechanism was termed stealth adaptation. It is envisioned as a generic process, which can potentially occur with all viruses [10,12].
A blood sample from the CFS patient from whom the initial stealth adapted virus was cultured had yielded a positive polymerase chain reaction (PCR) using low stringency conditions [10]. A slightly modified SK43 primer reactive with the tax gene of the human T lymphotropic virus I (HTLV-1) virus was used in combination with the SK44 primer, corresponding to a downstream tax gene sequence that is complementary to a sequence in HTLV-II [13]. This set of primers also yielded multiple PCR products when used on infected cells harvested from the virus cultures obtained from both the CFS and comatose patients. The PCR products were visualized as discrete bands on agarose gel electrophoresis. The PCR products were cloned into pBluescript plasmids and completely sequenced from the T3 and T7 promotor sites on the pBluescript plasmids. DNA sequencing of two large products from each culture that were amplified by the SK44 primer showed that each had a high degree of homology to SCMV. These results indicated that each virus had originated from SCMV [8,9]. The DNA sequence of a smaller PCR product generated by the SK43 primer from the culture obtained from the CFS patient is nearly identical to an intergenic region of the human X chromosome [14]. The entire DNA from the virus from the CFS patient was cloned and largely sequenced. While the sequences of many of the clones matched with a high degree of homology to SCMV, other clones showed either human cellular-related sequences or sequences of bacterial origin [15,16]. The same SK43, SK44 primer set gave variable results when tested on stealth adapted virus cultures from other CFS patients. The cultures were performed in commercially available human foreskin fibroblasts (MRHF cells). Occasional cultures would yield clearly definable PCR products as seen when analyzed using agarose gel electrophoresis. Several other tested cultures from CFS patients were completely negative by PCR using the same set of primers, even though the cultures clearly showed a strong CPE. Still other cultures would give weak DNA amplification, but the generated products were only easily detectable using a low stringency, dot blot hybridization assay. DNA sequencing was performed on a cloned agarose gel banded; SK43/SK44 generated PCR product obtained from a positive culture of an additional CFS patient. The sequencing showed that the amplified sequence had originated from the rhesus monkey genome [14]. To this article, this virus is designated stealth virus 3. PCR products from two additional and unrelated CFS patient, which could also be detected as bands in agarose gel electrophoresis, were also cloned and sequenced. These viruses are designated stealth virus-4 and stealth virus-5. Six cloned PCR products from the culture of stealth virus-4 and seven cloned PCR products from the culture of stealth virus-5 were partially sequenced. This article identifies the cellular origins of the virus sequences from stealth virus-4 and stealth virus-5. The results have important implications with regards to the potential infectious transmission of genetic illnesses.
Methods
The data relate to the analysis of DNA sequences of PCR products generated from positive stealth adapted virus cultures, obtained at different times from two unrelated CFS patients. The stealth viruses are designated as stealth virus-4 and stealth virus-5, respectively. The data also relate to a previously sequenced PCR product obtained from the stealth virus culture of another CFS patient. This virus is designated stealth virus 3. The virus cultures were established on MRHF human foreskin fibroblast cells using a vial of frozen-thawed mononuclear cells isolated from a 12ml heparinized blood sample from each patient. Serum-free X-Vivo medium was used in the cultures. A cytopathic effect (CPE) developed in each of the patients’ cultures. The CPE was transferable to additional cultures of MRHF cells. DNA was extracted from the cells following the appearance of the CPE. PCR was performed using a relatively low annealing temperature of 42 ℃ temperature for 40 cycles. The PCR primers were SK43, with a cytosine rather than an adenosine at the 10th nucleotide (5’-cggataccccgtctacgtgt-3’) and SK44 (5’-gagctgacaacgcgtccatcg-3’). The same set of primers had been used to amplify SCMV-related sequences in two previously cultured stealth adapted viruses, designated stealth virus 1 and stealth virus 2, respectively. An aliquot of the PCR generated material was analyzed using agarose gel electrophoresis. Another aliquot of the PCR generated material was treated with Klenow enzyme and phosphorylated before cloning into the pBluescript plasmid. The recombinant plasmids were grown in XL-1 bacteria. Partial sequencing of the clones was obtained by using the T3 and T7 promoter sites of the plasmids. The sequencing was performed at the City of Hope DNA sequencing facility using an Applied Biosystem 373 DNA analyzer. The DNA sequences beyond those of the PCR primers were analyzed using the BLASTN program of the National Center for Biotechnology Information (NCBI) [16]. The GenBank databases for DNA sequence analyses included the standard (nr/nt) collection, genomic + transcripts, and the latest assembled human, rhesus, and African green monkey genomes. The most closely matching GenBank sequences were determined for both the T3 and T7 readouts of each of the clones. The matching parameters included the default Expect Value (statistical significance to the power of e); the number of identical nucleotides over the length of the matching sequences; and the number of gaps that had to be inserted to obtain the optimal matching. The T3 and T7 matching cellular sequences were extended to include the intervening sequence, which was then subsequently analyzed for its likelihood of being either a protein coding or non-coding sequence using BLASTX program of the NCBI.
Results
Cloned PCR products generated using the SK43/SK44 set of primers on the culture of stealth virus-4 are designated as the C11 series and comprise six clones. The cloned PCR products obtained using the same set of primers at a different time on the culture of stealth virus-5 are designated as the C13 series and comprise seven clones. All the clones were partially sequenced using the T3 and T7 promoter sites on the pBluescript plasmids. The DNA sequences of the T3 and T7 readouts of both series of clones, including the primers, were submitted to the NCBI and each was assigned an accession number. These numbers begin with AF067469.1 for the T3 readout sequence of clone C1113 and extend to accession number AF067494.1 for the T7 readout of clone C1335. The DNA sequences beyond the primer sites were recently re-analyzed using the BLASTN program of the NCBI. For all of the sequences, the top GenBank matching was to either a rhesus monkey or a human genomic sequence. No sequence showed its preferred matching to the African green monkey genome or to any viral sequences. These BLASTN values allowed for the direct comparisons of the relative matching of each sequence with the rhesus and with the human genome. For the six clones from stealth virus 4, there is greater matching to the latest assembled genomic sequence of rhesus monkey (Mmul10) than to the latest assembled human genome (GRCh38.13). These comparisons are shown in Table 1. Similar comparisons were performed on the sequences of the seven clones of PCR products generated using the same set of primers on the culture of stealth virus 5. In this case, however, both the T3 and the T7 readouts of three of the clones (C1311, C1333, and C1334) show a greater homology to the assembled human compared to the assembled rhesus monkey genome. The T3 and T7 sequence readouts of the remaining four clones of the C13 series had sequences which matched more closely to the assembled rhesus monkey genome (Table 2).
Table 1: GenBank Matching to the Human (H) and Rhesus Monkey (RM) Assembled Cellular Genomes by the T3 and T7 Derived DNA Sequences of the PCR Products from the Culture of Stealth Virus-4.

1The clone number is followed by whether the sequence was read from the T3 or T7 promotor site on the pBluescript plasmid. This is followed by the NCBI accession number of the sequence. The number of nucleotides beyond those of the PCR primer is next shown, as is the range of the numbered nucleotides used in the BLASTN analysis.
Table 2: GenBank Matching to the Human (H) and Rhesus Monkey (RM) Assembled Cellular Genomes by the T3 and T7 Derived DNA Sequences of the PCR Products from the Culture of Stealth Virus-5.
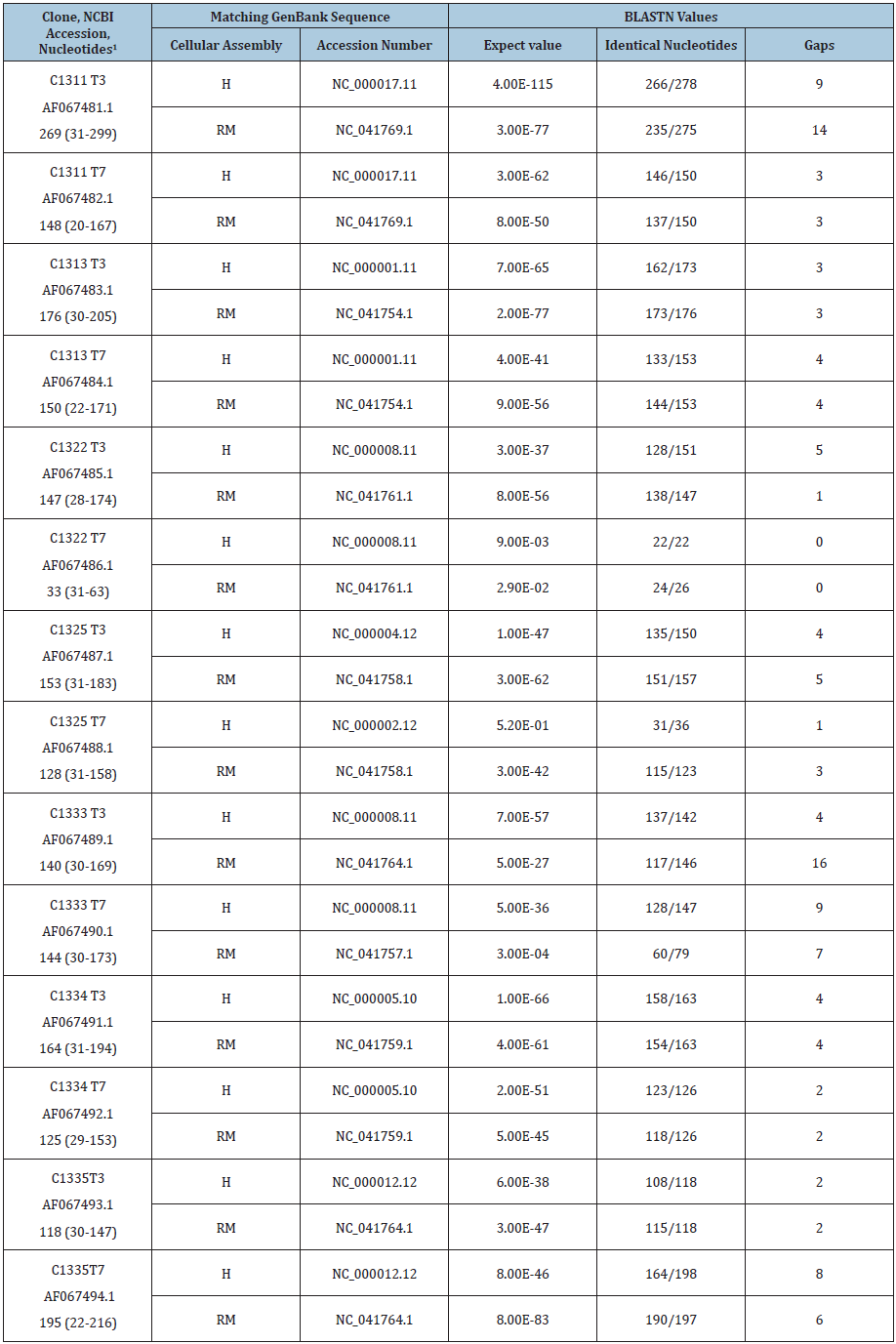
1The clone number is followed by whether the sequence was read from the T3 or T7 promotor site on the pBluescript plasmid. This is followed by the NCBI accession number of the sequence. The number of nucleotides beyond those of the PCR primer is next shown, as is the range of the numbered nucleotides used in the BLASTN analysis.
The sequences of two of the clones, C1113 and C1132, from the culture of stealth virus-4 and of one of the clones (C1322) from stealth virus-5, matched to the same region on chromosome 8 of the assembled rhesus monkey genome. The measures of the homology between the cloned sequences and that of the assembled rhesus monkey genome are slightly different from one another. Of interest, the sequences of the three clones also match closely to a fully sequenced clone generated using the same primer set on the culture from another CFS patient. The virus isolated from this patient is designated as stealth virus 3. This enabled a sequence comparison between the rhesus monkey genomic sequence, the fully sequenced PCR product from the culture of stealth virus-3, the matching partially sequenced PCR products of two clones generated from the culture of stealth virus-4, and the partially sequenced PCR products from a clone generated from the culture of stealth virus-5. An alignment of these five sequences is shown in Figure 1. The differences between one or more of the cloned sequences and the originating rhesus monkey genomic sequences comprise three types of changes. 1. Nucleotide additions (indicated by a dash inserted into the rhesus monkey genome sequence and a bold, but not underlined nucleotide in one or more of the cloned sequences in which a nucleotide addition has occurred). 2. Nucleotide deletion in one or more of the cloned sequences (indicated by a dash in the cloned sequence). 3. Nucleotide substitution in one or more of the cloned sequences (indicated by a bold and underlined nucleotide in the cloned sequence). Analysis of Figure 1 show several examples of each type of nucleotide change.
Figure 1: Alignments of the T3 and T7 Readouts of the Sequences of Clones C1113, C1132, and C1322 to the Rhesus Monkey Genome Sequence (NCBI Accession NC_041761.1) and to the PCR Generated Sequence From the Culture of Stealth Virus-3 (NCBI Accession AF107850.1)
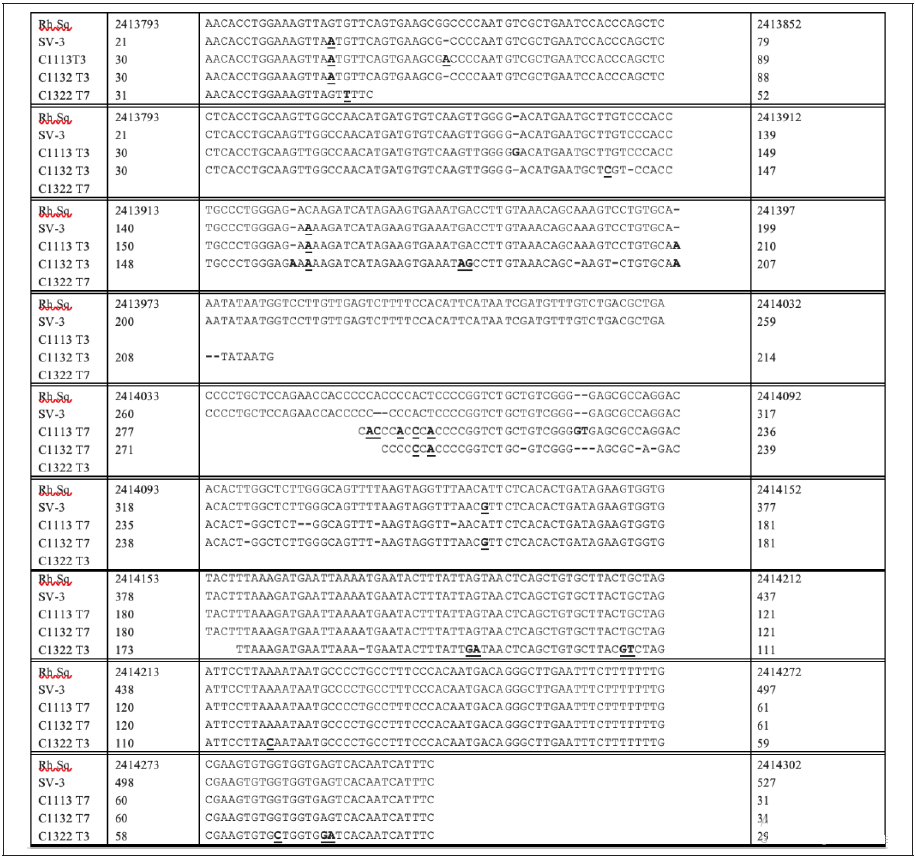
Footnote: The rhesus monkey sequence (Rh.Sq) is viewed as the primary sequence from which the other sequences have diverged. An inserted nucleotide in any of these other sequences requires that a dash (-) be inserted in the corresponding location in the Rh.Sq. The inserted sequence is shown in bold without underlining. A deleted nucleotide in the other sequences is also shown as a dash (-) in its sequence. Substituted nucleotides in the other sequences are shown as a bold and underlined nucleotide. SV-3 refers to stealth virus-3.
The T3 and T7 readouts from another of the clones from the culture of stealth virus-4 (C1123) matched to the same region of the rhesus monkey genome as did clone (C1313) from stealth virus 5. Figure 2 compares the sequences of the originating rhesus monkey genome with the T3 and T7 sequences of these two matching clones. Similarly, the T3 and T7 readouts from another of the clones from the culture of stealth virus-4 (C1142) matched to the same region of the rhesus monkey genome as did clone (C1335) from stealth virus-5. Figure 3 compares the sequences of the originating rhesus monkey genome with the T3 and T7 sequences of these two matching clones. The data displayed in Figures 2&3 again indicate occasional minor nucleotide additions, deletions, and substitutions. The originating sequences in the rhesus monkey genome, which best matched to the cloned T3 and T7 readouts of the C11 and C13 were extended to include the intervening sequences between the corresponding matching regions for the T3 and T7 readouts. The entire sequence was then analyzed using the BLASTX program of the NCBI. This program provides a comparison of the potentially translatable amino acid sequences with the protein database on GenBank. There were no protein matchings of the potentially translatable amino acid sequences for all but two of the identified genomic sequences. There was limited partial amino acid matching of the potentially translatable originating rhesus monkey genomic sequences identified by clones C1151 and C1325. The highest level of minor partial matching of each of the two translated amino acid sequences were, however, to different bacterial proteins, rather than to cellular proteins (data not shown).
Figure 2: Alignments of the T3 and T7 Readouts of the Sequences of Clones C1123 and C1313 to the Rhesus Monkey Genome Sequence (NCBI Accession NC_041754.1)
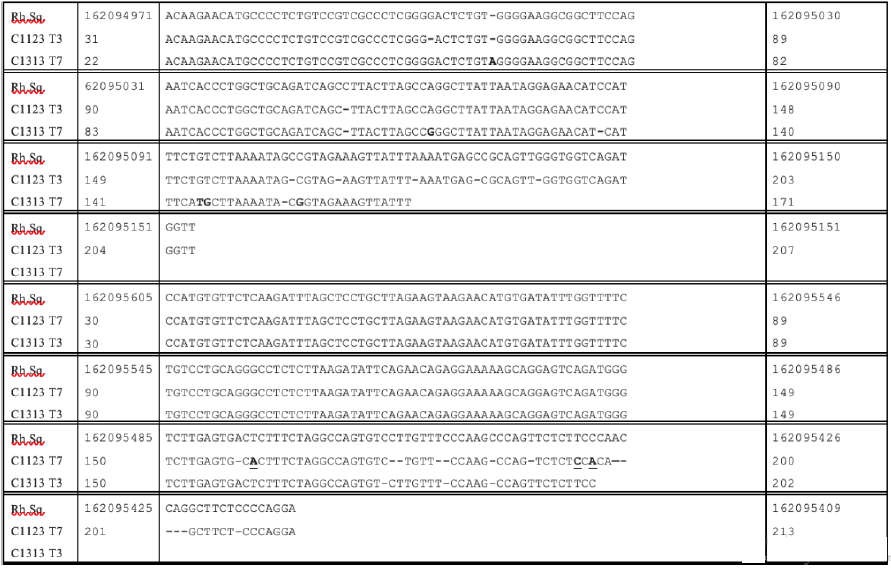
Footnote: The rhesus monkey sequence (Rh.Sq) is viewed as the primary sequence from which the other two sequences have diverged. An inserted nucleotide in any of these two sequences requires that a dash (-) be inserted in the corresponding location in the Rh.Sq. The inserted sequence is shown in bold without underlining. A deleted nucleotide in one or both of the other two sequences is also shown as a dash (-) in the sequence. Substituted nucleotides in the other two sequences are shown as a bold and underlined nucleotide.
Figure 3: Alignments of the T3 and T7 Readouts of the Sequences of Clones C1142 and C1335 to the Rhesus Monkey Genome Sequence (NCBI Accession NC_041764.1)
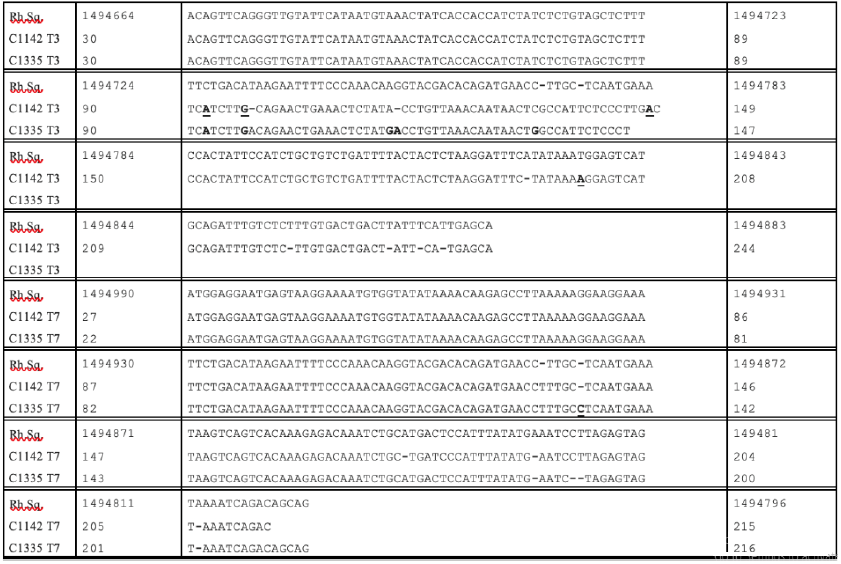
Footnote: The rhesus monkey sequence (Rh.Sq) is viewed as the primary sequence from which the other two sequences have diverged. An inserted nucleotide in any of these two sequences requires that a dash (-) be inserted in the corresponding location in the Rh.Sq. The inserted sequence is shown in bold without underlining. A deleted nucleotide in one or both of the other two sequences is also shown as a dash (-) in the sequence. Substituted nucleotides in the other two sequences are shown as a bold and underlined nucleotide.
Discussıon
As shown in this article, the sequences in the PCR products generated in the virus cultures of two CFS patients are cellular in origin, with significant subsequent minor genetic mutations. For the culture of stealth virus 4, all of the detected sequences originated from the rhesus monkey genome. The sequences in the culture of stealth virus-5 comprise four that are of rhesus monkey genome origin and three that originated from the human genome. The human cellular sequences have presumably substituted for monkey cellular sequences. This is likely to have occurred by homologous recombination. This is consistent with a prior observation that the cellular sequences detected in a SCMV originating stealth adapted are also from the human genome [14,15]. Because of homologous recombination it is possible that some of the originating monkey cellular sequences could recombine into the human chromosome. Were this to occur in a germ cell, the inserted sequences could be maintained in future generations, reflecting as form of reverse evolution. Although slightly divergent from the originated rhesus monkey genomic sequence, the cellular-derived sequence of the PCR product amplified from stealth virus-3, match closely to the T3 and T7 readout sequences of two cloned PCR products from the culture of stealth virus-4 and to the T3 and T7 readout sequences of a cloned PCR product from the culture of stealth virus-5. Similarly, stealth virus cultures 4 and 5 yielded two additional sets of T3 and T7 matching, but non-identical monkey cellular-derived sequences. These data are consistent with the continuing replication and a widespread person-to-person transmission of originally cellular-derived genetic sequences, which are undergoing further mutations.
Agarose gel electrophoresis analysis of the DNA isolated from the prototypic stealth adapted SCMV showed that most of the virus DNA comprised fragments of approximately 20 kilobases (kb) [10,17,18]. Yet the total unique nucleotide content well exceeds 100kb [12]. If genomic fragmentation is generally true for other stealth adapted viruses, than a proposed mechanism for the incorporation of cellular sequences into a stealth adapted virus would be via the linking of portions of the fragmented originating virus genome with cellular RNA sequences. This is supported by unpublished data on a partially sequenced clone obtained from the prototype SCMV-derived stealth adapted virus. One end of the clone has an SCMV-related sequence while the other end has a cellular-derived sequence. The formation of DNA from RNA further implies a role for endogenous reverse transcriptase enzymes in the replication of the incorporated cellular sequences. It would not be surprising, therefore, if some future detected cellular sequences in stealth adapted viruses are related to endogenous reverse transcriptase. In a previously cultured stealth adapted virus, the SCMV-reactive PCR assay required the prior use of an added reverse transcriptase [19]. This raises the additional possibility of an originating DNA stealth adapted virus becoming a replicating RNA virus. To date, none of the identified cellular sequences correspond to protein coding regions of cellular genes. As discussed in an earlier article, protein coding genetic sequences from bacteria have been detected in the prototypic SCMV-derived stealth adapted virus [14,16]. These proteins may serve as the equivalence of capsid proteins in certain stealth adapted viruses. Using a single set of PCR primers is clearly insufficient to fully characterize a cytopathic virus. Nevertheless, the results are sufficient to conclude that cellular-derived sequences can become component parts of stealth adapted viruses. While subject to ongoing mutations and homologous recombination, the incorporated cellular sequences can be maintained throughout the virus replication and transmission process. While this has been known to have occurred during the ancestry of many of the larger human viruses [20], the concept of an active ongoing present-day process is novel. Indeed, it can change the perception of viruses as being genetically distinct from eukaryotic cells and from bacteria. Rather, stealth adapted viruses can seemingly allow for the ready transmission of cellular sequences between species. The term renegade cellular sequence has been introduced to highlight the altered fate of a genomic sequence upon its incorporation into a replicating and transmissible virus [14].
This process has major implications with regards to human and animal health. In excluding a virus cause of an illness, it is no longer adequate to simply fail to detect serological and genetic markers of known human and animal viruses. Thus, viruses can potentially be disguised as unstable cellular sequences. The detection of a CPE in virus cultures provides a sensitive screening assay for stealth adapted viruses, which may go undetected using conventional virus-reactive molecular or antibody probes. CFS and autism are two prominent examples of illnesses in which stealth adapted viruses can be reliably detected using specialized culture methods [9,10,19, 21-23]. Yet viruses have not been detected in these illnesses by other virologists; or have been falsely identified [24,25]. Stealth adapted viruses have been detected in the blood and cerebrospinal fluids of patients with a wide range of neurological and psychiatric illnesses [9,10,19, 21-23]. The brain is particularly susceptible to symptomatic illness caused by stealth adapted viruses. Organs with overall uniform cellular activity, such as the liver, kidney, and lung, can compensate for limited localized cellular damage due to stealth adapted viruses. This does not apply to the brain with its complex regional networking. It also does not apply to the skin in which illnesses, such as vitiligo, can reflect localized damage to melanocytes. Cancer is another illness in which the disease can occur from a single genetically damaged cell. It was not anticipated by public health authorities that the emergence of stealth adapted viruses would become an adverse consequence of the production of virus vaccines. Unlike virus infectious with overt severe clinical manifestations, illnesses characterized by subtle and diverse impairments in brain function are difficult to relate to an infectious process. This is particularly so if there is genetic heterogenicity of the infectious agents and an absence of inflammation, as with stealth adapted viruses. Unfortunately, there has been additional pushback by public health authorities to the concept of stealth adapted viruses. This has occurred in part because of the linkage of the experimental use of poliovirus vaccines in chimpanzees with the emergence of the human immunodeficiency virus (HIV) [26]. The potential of future long-term consequences of using animal cells to produce vaccines for use in humans should be of sufficient concern to public health authorities to abolish this practice.
Efforts are also needed to assess the extent to which humans have become infected with stealth adapted viruses and to catalogue the potentially transmissible aberrant cellular sequences incorporated into these viruses. Unique and/or mutated non-coding RNA sequences have been linked with certain specific illnesses [27]. Were such sequences to be conveyed between humans via stealth adapted viruses, it would lead to an increasing incidence of the resulting illness. These should, therefore, be a heightened awareness of the potential transmissibility of various genetic disorders from the incorporation of the genes responsible for these known genetic disorders into stealth adapted viruses. In other words, this would be an increased incidence of an illness that was previously considered to be purely genetic in origin. In many ways, the increasing incidence of autism may reflect this change. The identification of stealth adapted viruses has been useful in revealing a potent non-immunological anti-virus defense mechanism mediated by the alternative cellular energy (ACE) pathway [28-33]. This pathway is reflected in an added dynamic or kinetic property of the body’s fluids. As currently proposed, the fluids are activated by an environmental force termed KELEA, an abbreviation for Kinetic Energy Limiting Electrostatic Attraction. The brain and possibly muscles are thought to act as the primary receivers of KELEA and its transfer to the body fluids. The ACE pathway is a source of cellular energy which can enable cells to respond to virus infections more effectively. Enhancing the ACE pathway is effective in the suppression of both stealth adapted and conventional viruses [32,33].
In summary, two stealth adapted viruses cultured from CFS patients were shown by PCR to have several unstable genetic sequences which had originated from non-coding regions of the rhesus monkey genome. One of the stealth adapted viruses had seemingly exchanged three monkey-derived cellular sequences for human genome-derived sequences, presumably by homologous recombination. These findings extend the concept of stealth adaptation beyond the deletion or mutation of the genes coding for the relatively few virus components that are normally targeted by the cellular immune system. As previously proposed, stealth adaptation can include the substitution of some of the originating virus sequences with genetic sequences from the infected cells. This may be a consequence of the fragmentation of the stealth adapted virus genome allowing for the linear combining of overlapping sequences of originating virus fragments with transcribed cellular RNA sequences. Incorporated monkey genome derived cellular sequences can be retained in the stealth adapted viruses as they are transmitted from cultured monkey cells to humans and subsequently transmitted between humans. As carriers of mutated and genetically unstable animal and human genetic sequences, stealth adapted viruses pose a major risk for transmissible genetic illnesses in humans. The findings should lead to the termination of using animal cells in the production of vaccines for use in humans. Studies are also indicated to determine the extent of prior contamination of humans with transmissible mutated animal and human derived genomic sequences.
Acknowledgment
The work was supported by MI Hope Inc, a non-profit public charity. This article with a slight change in the Title has also been published in the Journal of Human Virology and Retrovirology. Duplication is intended and agreed upon to help reach as wide a readership as possible given the significance of infectious monkeyderived cellular sequences being transmitted to humans.
Human Investigation
The culturing of viruses and the obtaining of DNA sequence data on positive virus cultures were approved by the Los Angeles County + University of Southern California Institutional Review Board. The investigational testing was performed in a CLIA Certified Laboratory with the Informed Consent of those tested. No personal identifying information regarding the virus culture positive individuals is included in the manuscript.
Availability of Data
All cited DNA data are deposited at NCBI GenBank.
References
- Barone PW, Wiebe ME, Leung J (2020) Viral contamination in biologic manufacture and implications for emerging therapies. Nat Biotechnol 38: 563-572.
- Robbins FC (1999) The history of polio vaccine development. pp. 13-27.
- Bookchin D, SchumacherJ (2004) The virus and the vaccine: the true story of a cancer-causing monkey virus, contaminated polio vaccine, and the millions of Americans exposed. St. Martin's Press pp. 380
- Sweet BH, Hilleman MR (1960) The vacuolating virus, SV40. Proc Soc Exp Biol Med 105: 420-427.
- Eddy BE, Borman GS, Grubbs GE, Young RD (1962) Identification of oncogenic substance in rhesus monkey kidney cell cultures as simian virus 40. Virology 17: 65-75.
- Yue Y, Kaur A, Lilja A, Diamond DJ, Walter MR (2016) The susceptibility of primary cultured rhesus macaque kidney epithelial cells to rhesus cytomegalovirus strains. Journal of General Virology97(6): 1426-1438.
- Smith KO, Thiel JF, Newman JT, Harvey E, Trousdale MD, et al. (1969) Cytomegaloviruses as common adventitious contaminants in primary African green monkey kidney cell cultures. JNCI: Journal of the National Cancer Institute 42(3): 489-496.
- Martin WJ, Ahmed KN, Zeng LC, Olsen JC, Seward JG, et al. (1995) African green monkey origin of the atypical cytopathic 'stealth virus' isolated from a patient with chronic fatigue syndrome. Clin Diagn Virol 4(1): 93‐103.
- Martin WJ (1996) Simian cytomegalovirus‑related stealth virus isolated from the cerebrospinal fluid of a patient with bipolar psychosis and acute encephalopathy. Pathobiology 64: 64‑66.
- Martin WJ, Zeng LC, Ahmed K, Roy M (1994) Cytomegalovirus‑related sequences in an atypical cytopathic virus repeatedly isolated from a patient with the chronic fatigue syndrome. Am J Path 145: 441‑452.
- Martin WJ, Glass RT (1995) Acute encephalopathy induced in cats with a stealth virus isolated from a patient with chronic fatigue syndrome. Pathobiology 63: 115‑118.
- Martin WJ (2014) Stealth adaptation of viruses: Review and updated molecular analysis on a stealth adapted African green monkey simian cytomegalovirus (SCMV). J Human Virology & Retrovirology 1(4): 00020.
- Ehrlich GD, Glaser JB, LaVigne K (1989) Prevalence of human T-cell leukemia lymphoma virus (HTLV) type II infection among high-risk individuals: Type-specific identification of HTLVs by polymerase chain reaction. Blood 74(5): 1658.
- Martin WJ (2019) Renegade cellular and bacterial genetic sequences in monkey-derived stealth adapted viruses. J Human Virology & Retrovirology 7(2): 26-40.
- Martin WJ (1998) Cellular sequences in stealth viruses. Pathobiology 66: 53-58.
- Martin WJ (1999) Bacteria related sequences in a simian cytomegalovirus-derived stealth virus culture. Exp Mol Path 66: 8-14.
- Hu G, Kurgan L (2019) Sequence similarity searching. Curr Protoc Protein Sci 95(1): e71.
- Martin WJ (1996) Genetic instability and fragmentation of a stealth viral genome. Pathobiology 64: 9‑17.
- Martin WJ (1997) Detection of RNA sequences in cultures of a stealth virus isolated from the cerebrospinal fluid of a health care worker with chronic fatigue syndrome. Case report. Pathobiology 65: 57-60.
- Krupovic M, Koonin EV (2017) Multiple origins of viral capsid proteins from cellular ancestors. Proc Natl Acad Sci 114(12): E2401-E2410.
- Martin WJ (1995) Stealth virus isolated from an autistic child. J Aut Dev Dis 25: 223‑224.
- Martin WJ (1996) Severe stealth virus encephalopathy following chronic fatigue syndrome‑like illness: Clinical and histopathological features. Pathobiology 64: 1‑8.
- Martin WJ (1996) Stealth viral encephalopathy: Report of a fatal case complicated by cerebral vasculitis. Pathobiology 64: 59‑63.
- DeFreitas E, Hilliard B, Cheney PR (1991) Retroviral sequences related to human T-lymphotropic virus type II in patients with chronic fatigue immune dysfunction syndrome. Proc Natl Acad Sci USA 88(7): 2922‐2926.
- Alter HJ, Mikovits JA, Switzer WM (2012) A multicenter blinded analysis indicates no association between chronic fatigue syndrome/myalgic encephalomyelitis and either xenotropic murine leukemia virus-related virus or polytropic murine leukemia virus. MBio 3(5): e00266-12.
- Martin WJ (2015) Chimpanzees inoculated with cytomegalovirus contaminated polio vaccines may explain origin of HIV-1. J Human Virology & Retrovirology 2(2): 00035.
- Yao RW, Wang Y, Chen LL (2019) Cellular functions of long noncoding RNAs. Nat Cell Biol 21(5): 542‐551.
- Martin WJ (2003) Stealth virus culture pigments: A potential source of cellular energy. Exp Mol Path 74: 210-223.
- Martin WJ (2014) Stealth adapted viruses; alternative cellular energy (ACE) & KELEA activated water. Author House Bloomingdale pp. 3-30.
- Martin WJ (2016) Deconstructing medicine: The alternative cellular energy pathway. British J Medicine & Medical Research 11(8): 1-6.
- Martin WJ (2016) Insufficiency of cellular energy (ICE) the basis for many illnesses potentially correctable using KELEA activated water. International J Complementary & Alternative Medicine 4(1): 00106.
- Martin WJ (2016) The ACE pathway in comparison to the immune system in the defense against infectious diseases. J Human Virology & Retrovirology 3(5): 00124.
- Martin WJ (2020) Enhancing the alternative cellular energy (ACE) pathway with KELEA activated water as therapy for infectious diseases. Infectious Disorders Drug Targets 20(1): 1-6.
© 2020 W John Martin. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






