- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Evaluation of Advansure TB/NTM Real Time PCR Kit for Detection of Mycobacteria in Sputum Specimens
Gülnur Tarhan1*, Hülya Şimşek2 and Ismail Ceyhan3
1Faculty of Medicine, Departmenf of Medical Microbiology, Turkey
2Faculty of Medicine, Departmenf of Medical Microbiology, Turkey
3Faculty of Medicine, Departmenf of Medical Microbiology, Turkey
*Corresponding author: Gülnur Tarhan, Faculty of Medicine, Department of Medical Microbiology, Turkey
Submission: November 20, 2019; Published: July 17, 2020

ISSN 2578-0190 Volume4 issues1
Abstract
Real time PCR is a widely used for molecular diagnosis of tuberculosis in routine diagnosis laboratory. There are many commercially avaliable system. The sensitivity of the system varies according to DNA extraction from the sample and target specific primers and probes and commercial systems. In this study, the sensitivity and specificity of AdvanSure TB/NTM real time PCR kit were evaluated for detecting mycobacteria in smear positive and negative sputum specimens of 35 active pulmonary tuberculosis patients. All specimens were corrected using EZN staining, solid (Löwenstein Jensen) and liquid culture (MGIT). Culture and clinical findings were used as gold standards in the evaluation of all PCR results. Overall true positivity was found 25 ( 71.42%). When smear negative samples were evaluated together with culture results, only 3 patients were found positive by PCR test in 10 samples (30%).
Keywords: Mycobacterium tuberculosis; Non-tuberculous mycobacterium; AdvanSure TB/NTM; real-time PCR
Introduction
Tuberculosis caused by Mycobacterium tuberculosis complex (MTBC) is still one of the most wide-spread and serious infectious diseases all over the world. Despite successful treatment and control measures, approximately 10 million people are affected by tuberculosis each year. In addition, AIDS, cancer, intravenous drug addiction and so on [1,2]. Other mycobacterial infections, which are found as saprophytes, are increasing day by day due to immunosuppressive causes. Over 160 species that have not been found in the Mycobacterium tuberculosis complex have been reported, which are called non-tuberculosis mycobacteria (NTM) or mycobacteria other than tuberculosis (MOTT) [3,4]. The most common species causing disease is called Mycobacterium avium complex. The next most common are Mycobacterium abscessus complex and Mycobacterium kansasii [5]. The incidence of tuberculosis caused by NTM species is not much common, although observed in some African regions [6,7]. Infections caused by NTMs tend to increase gradually due to the above mentioned causes of immunosuppression. Some studies have indicated an increase in the frequency of NTM isolation from respiratory specimens in worldwide [8-11]. Due to the different treatment procedures and duration, early diagnosis and identfication of MTBC and NTM is the most important stage before starting treatment. Primary diagnosis of tuberculosis is made by history, chest X - ray, smear microscopy, PPD or gamma interferon release test. Smear microscopy is the simplest and cheapest method known. However, the sensitivity of the test is low. This test does not distinguish between MTBC and NTM. The detection of MTBC in sputum culture is considered the gold standard in the diagnosis of tuberculosis. The detection of MTBC in sputum culture is considered the gold standard in the diagnosis of tuberculosis. However, a long incubation period of 2-8 weeks should be waited for the definitive result by culture method. Molecular diagnostic tests for the detection of MTBC provide the possibility of diagnosis in a short time. But their sensitivity varies according to the system used. The detection of MTBC in sputum culture is considered the gold standard in the diagnosis of tuberculosis. However, a long incubation period of 2-8 weeks should be waited for the exact result by culture method. Species-level identification with conventional methods is time consuming. Mostly, sensitive results cannot be obtained. Only NMT and MTBC can be distinguished by card-based tests used in species identification. Molecular-based tests used for this purpose require both expensive and special equipment. Real time PCR is a widely used for routine molecular diagnosis of pulmonary and extrapulmonary tuberculosis in clinical laboratories. There are many commercially avaliable system. The sensitivity of the system varies according to DNA extraction from the sample and target specific primers and probes and commercial systems [12-16]. AdvanSure TB/NTM real time PCR kit is a new diagnostic real-time PCR test for Mycobacterium tuberculosis complex and NTM. In this system; during the real time PCR reaction, MTB and NTM can be developed for rapid MTB detection and NTM discrimination [17,18]. The aim of this study was to assess the diagnostic performance of AdvanSure TB/NTM real time PCR kit smear positive and negative sputum specimens. All specimens were corrected using EZN staining, solid (Löwenstein Jensen) and liquid culture (MGIT).
Materials and Methods
Specimen groups
25AFB (+) and 10 AFB(-) patients diagnosed with active pulmonary tuberculosis through clinical findings, radiology and laboratory investigations, and 5 healty controls and 10 reference strains (mycobacterium tuberculosis, mycobacterium gordonae, mycobacterium kansasii, mycobacterium chelonae, mycobacterium intracellulare, mycobacterium scrofulaceum, mycobacterium abscessus, mycobacterium xenopi, mycobacterium smegmatis, mycobacterium africanum)were evaluated .All clinical samples were verified with microscopy, culture.
Specimen preparation [19]
Sputum samples were decontaminated by treatment with an equal volume %4 NaOH trisodium citrate N -acetyl-L-cysteine ( NALC) for 15 min at room temperature and were neutralized with sterile 0.067M phosphate buffer (pH : 6.8). After centrifugation at 3,000 X g for 15 min, each pellet was resuspended in 1.0 ml sterile 0.067 M phosphate buffer (pH : 6.8). 1ml aliquot of the suspension was inoculated onto Löwenstein-Jensen( LJ ) culture media and used for acid fast staining. Slopes was incubated at 37 °C. Slopes were inspected weekly for up to 8 weeks. Fixed smears were stained Erlich- Ziehl- Neelsen ( EZN) staining. All slides were confirmed to be positive or negative by microscopy. The remainder of aliquot was directly processed for PCR or kept at –20 0C until used.
Reference strains
A loopful bacteria were transferred to glass bead tubes containing 3 ml 0.9 % NaCl from culture .With 15 min vortex, bacteria was seperated as much as possible suspension prepared were adjusted according to Mac Farland 1. And 3X108 mycobacteria were yielded in 1 ml suspension was measured at 625nm in spectrophotometre and adjusted so as to have a absorbance of 0.177. 1 ml of this suspension was mixed with 2 ml.0.9 % NaCl and 1ml suspension and 108 mycobacteria were obtained. From this main suspension, 102 and 101 dilutions were obtained by serial dilutions. They were kept at –20 0C until used.
DNA extraction
All samples were prepared according to the instructions of the manufacturer(AdvanSure TB/NTM real-time PCR kit (LG Lifescience, Seoul, Korea).
PCR reactions
The AdvanSure TB/NTM real-time PCR kit (LG Lifescience, Seoul, Korea) with the SLAN real-time PCR detection system (LG Lifescience), and the Real-Q mycobacterium tuberculosis kit (Biosewoom, Seoul, Korea) with the Rotor-Gene Q (Corbett Life Science, Sydney, Australia), for the real-time PCR analysis.
Results and Discussion
Table 1:
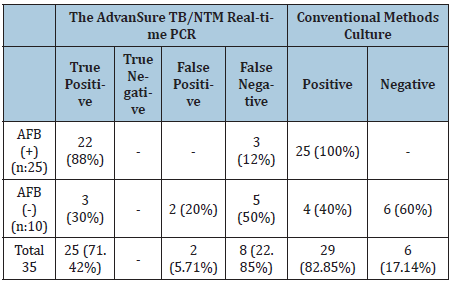
Culture and clinical findings were used as gold standards in the evaluation of all PCR results. Overall true positivity was found 25 ( 71.42%). Of the 25 smear-positive samples, 22 were found positive (88%) by PCR. Three smear positive samples showed negative results by PCR test. The clinical findings and culture results of these samples were positive. 3 of the 10 smear negative sputum samples suspected of tuberculosis were found positive (30%) by PCR test. The culture result of 2 of these samples was positive . PCR result of a sample with positive smear negative culture was found as negative. All data for the study are shown in Table 1. PCR results of sputum samples of 5 healthy individuals evaluated in the study were negative. No cross-reactions were observed between the reference 10 reference strains (mycobacterium tuberculosis, mycobacterium gordonae, mycobacterium kansasii, mycobacterium chelonae, mycobacterium intracellulare, mycobacterium scrofulaceum, mycobacterium abscessus, mycobacterium xenopi, mycobacterium smegmatis, mycobacterium africanum) evaluated in the study.
Discussıon
PCR systems which are prepared by using various technologies are used in routine diagnostic laboratories as a support to microscopy and culture methods in the diagnosis of tuberculosis. AdvanSure TB / NTM real time PCR system is a molecular diagnostic method developed for rapid diagnosis and identification of TB / NTM in smear positive samples. There is limited data on the sensitivity and specificity of this test in smear positive and smear negative samples. The aim of this study was to evaluate the diagnostic value of AdvanSure TB / NTM real time PCR system in patients with smear positive and smear negative patients with suspected pulmonary tuberculosis and clinical and radiological findings. In our study, the sensitivity of AdvanSure TB / NTM real time PCR system was found to be 88% in smear positive. In this study, similar results were observed with previous studies (70.9%-88.7%) [20-23]. In our study, since the number of smear negative patients was low, no significant results were obtained in sensitivity, specificity, positive and negative predictive values. True positive detection rate was found as 71.42% in all sample groups. In the diagnostic algorithm published by the CDC, PCR tests are only recommended for microscopic confirmation of smear-positive samples and for the precise identification of the causative agent Mycobacterium tuberculosis [24]. When smear negative samples were evaluated together with culture results, only 3 patients were found positive by PCR test in 10 samples (30%). Although 5 patients had positive culture results, PCR test was negative. These patients were thought to have strong tuberculosis due to clinical, radiological and other laboratory findings and consisted of patients who were treated for tuberculosis. The number of patients we examined in the smear negative patient group was insufficient and there was not enough knitting group in the definitive evaluation of the PCR test. Therefore, we believe that a larger patient population should be evaluated for the test. One of the most important features of this test is the ability to distinguish between TB and NTM. In the sputum samples of 35 smear positive and smear negative patients that we used for the test, all of the samples detected positive by PCR test were identified as TB. Mycobacterium tuberculosis ve Mycobacterium tuberculosis and 9 NTM (mycobacterium gordonae, mycobacterium kansasii, mycobacterium chelonae, mycobacterium intracellulare, mycobacterium scrofulaceum, mycobacterium abscessus, mycobacterium xenopi, mycobacterium smegmatis, mycobacterium africanum) bacterial suspensions showed no cross-reaction between the sample groups. As a result, the AdvanSure TB / NTM real time PCR system can be used in coordination with microscopy to differentiate TB diagnosis and NTM, especially in smear positive patients. Further studies are needed in smear negative samples. The ability to discriminate between TB and NTM simultaneously with this test appears to be a significant advantage in the formulation of the causative treatment protocol.
References
- World Health Organization.
- Adam MacNeil, Philippe Glaziou, Charalambos Sismanidis, Susan Maloney, Katherine Floyd (2019) Global epidemiology of tuberculosis and progress toward achieving global targets. Weekly 68(11): 263-266.
- (1997) Diagnosis and treatment of disease caused by nontuberculous mycobacteria This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med 156(2): S1-S25.
- Johnson MM, Odell JA (2014) Nontuberculous mycobacterial pulmonary infections. J Thorac Dis 6(3): 210-220.
- What is Nontuberculous Mycobacteria (NTM) Disease?
- Niemann S, Rusch GS, Joloba ML (2002) Mycobacterium africanum subtype II is associated with two distinct genotypes and is a major cause of human tuberculosis in Kampala, Uganda. J Clin Microbiol 40: 3398-3405.
- Kwon YS, Koh WJ (2016) Diagnosis and treatment of nontuberculous Mycobacterial lung disease. J Korean Med Sci 31: 649-659.
- Koh WJ, Kwon OJ, Lee KS (2005) Diagnosis and treatment of nontuberculous mycobacterial pulmonary diseases: a Korean perspective. J Korean Med Sci 20: 913-925.
- Koh WJ, Kwon OJ, Jeon K (2006) Clinical significance of nontuberculous mycobacteria isolated from respiratory specimens in Korea. Chest 129: 341-348.
- Park YS, Lee CH, Lee SM (2010) Rapid increase of non-tuberculous mycobacterial lung diseases at a tertiary referral hospital in South Korea. Int J Tuberc Lung Dis 14: 1069-1071.
- Diagnostic standards and classification of tuberculosis (1990) American Thoracic Society Am Rev Respir Dis 142: 725-735.
- Diagnosis of Mycobacterium tuberculosis.
- Laraque F, Griggs A, Slopen M, Munsiff SS (2009) Performance of nucleic acid amplification tests for diagnosis of tuberculosis in a large urban setting. Clin Infect Dis 49: 46-54.
- Medium selection and incubation for the isolation of Mycobacteria.
- Balasingham SV, Davidsen TI, Szpinda SA, Tonjum T (2009) Molecular diagnostics in tuberculosis: basis and implications for therapy. Mol Diagn Ther 13: 137-151.
- Lee H, Park KG, Lee G, Park J, Park YG, et al. (2014) Assessment of the quantitative ability of AdvanSure TB/NTM real-time PCR in respiratory specimens by comparison with phenotypic methods. Ann Lab Med 34(1): 51‐
- Hwang S, Oh KJ, Jang IH (2011) Evaluation of the diagnostic performance of the AdvanSure TB/NTM Real-Time PCR kit for detection of mycobacteria. Ann Clin Microbiol 14(2): 55‐
- Kubica GPW, Dye E, Cohn ML, Middlebrook G (1963) Sputum digestion and decontamination with N-acetyl-Lcysteine- sodiumhydroxide for culture of mycobacteria. Am Rev Respir Dis 87: 775-779.
- Choe W, Kim E, Park SY, Chae JD (2015) Performance evaluation of anyplex plus MTB/NTM and AdvanSure TB/NTM for the detection of Mycobacterium tuberculosis and nontuberculous mycobacteria. Ann Clin Microbiol 18(2): 44‐
- Cho WH, Won EJ, Choi HJ (2015) Comparison of AdvanSure TB/NTM PCR and COBAS TaqMan MTB PCR for detection of Mycobacterium tuberculosis complex in routine clinical practice. Ann Lab Med 35(3): 356‐
- Lim JH, Bae MH (2019) Evaluation of the performance of two real-time PCR assays for detecting Mycobacterium species. J Clin Lab Anal 33: e22645.
- Choe W, Kim E, Park SY, Chae JD (2015) Performance evaluation of Anyplex plus MTB/NTM and AdvanSure TB/NTM for the detection of Mycobacterium tuberculosis and nontuberculous mycobacteria. Ann Clin Microbiol 18: 44-51.
- Updated guidelines for the use of nucleic acid amplification tests in the diagnosis of tuberculosis.
© 2020 Gülnur Tarhan. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






