- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Global Clinical Epidemiology of Carbapenem- Resistant Enterobacteriaceae Bacteremia and Association with Mortality: Systematic Review and Meta-Analysis
Wu Menglu¹, Lin Qiuxia¹, Zhong Hong¹, Zou Hua¹ and Huang Shifeng²*
1The First Clinical College, Chongqing Medical University, Chongqing, China
2Department of Laboratory Medicine, the First Affiliated Hospital of Chongqing Medical University, Chongqing, China
*Corresponding author: Shifeng Huang, Department of Laboratory Medicine, China
Submission: February 07, 2020; Published: February 20, 2020

ISSN 2578-0190 Volume3 issues4
Abstract
Objectives: This study was aimed to systematically review published data to evaluate the clinical epidemiology, to explore the risk factors for the acquisition of CRE bacteremia among hospitalized patients and to find out their association with mortality.
Methods: The reports concerning the CRE bacteremia in hospitalized adult patients among the published literature before May 2019 were identified by a systematic search of Pubmed, EMBASE and Cochrane. Summary odds ratios(OR) were calculated using random effects models, and study quality was assessed using a modified Newcastle-Ottawa scale.
Results: Totally 573 literatures were retrieved out, and we identified 42 studies to calculate the statistically significant pooled odds ratio, of which 22 papers describing factors for CRE-BSIs morbidity and 26 papers for mortality. Previous antibiotic exposure (OR 7.71; 95% CI 2.82-21.08; I-squared=87%), following by mechanical ventilation (OR 4.54; 95% CI 2.55, 8.08; I-squared=78%) and admission to ICU (OR 4.17; 95% CI 3.02-5.76; I-squared=72%) device generated the highest pooled estimate for CRE-BSIs morbidity. Underlying diseases or conditions lead to an unfortunate ending for patients with CRE-BSI. Appropriate empirical therapy contributed to reduce mortality for CRE-BSIs, and the use of ceftazidime-avibactam, lower Pitt bacteremia score or APACHE2 score were also relevant to control mortality.
Conclusion: The worldwide morbidity and mortality for CRE-BSIs are high. We should standardize medical practices, optimize the therapeutic approach, timely monitor relevant indicators to control hospital outbreaks.
Keywords: Carbapenem; Resistant; Enterobacteriaceae; Blood stream infection; Risk factors; Meta-analysis
Introduction
Carbapenems have traditionally been considered as the drugs of last resort for Gram-negative bacterial infections [1]. Unfortunately, outbreaks due to Carbapenem-Resistant Enterobacteriaceae (CRE) have been reported in many countries around the world, such as Europe, Asia, South America, and certain regions of North America [2]. It has become a global public health threat in the past decade, with the prevalence rate increased from 1.2% in 2001 to 4.2% in 2011 as reported by the Centers for Disease Control and Prevention in the United States [3]. The use of antibiotics has accelerated the emergence of CRE, because of the cross transmission and infection control measures, leading to adverse, even life-threatening outcomes, in patients with hospital acquired CRE infections. As the severest kind of CRE infection, CRE blood stream infection (CRE-BSI) is usually associated with poor prognosis, prolonged hospital stays, and extremely considerable mortality due to the paucity of effective treatment options [4]. The mortality rates for CRE bacteremia ranged from 40% to 60% as was observed from the United States, Italy, Greece, and Spain [5]. As was reported by Matthew E patients with CRE bacteremia showed 2-fold higher unadjusted number of deaths than those with CSE-infected patients [6]. Nevertheless, there have been only few large-scale studies which thoroughly and comprehensively analyzed risk factors for CRE bacteremia. Moreover, high variability was shown due to different geographical locations, and several risk factors for CRE bacteremia have been revealed in retrospective studies with inconsistent conclusions. To prevent further increase in CRE bacteremia infections by improving infection prevention strategies, this study was aimed to systematically review published data to evaluate the clinical epidemiology, to explore the risk factors for the acquisition of CRE bacteremia among hospitalized patients and to find out their association with mortality. Furthermore, we are going to figure out the essential components of effective infection control in preventing or ending hospital CRE bacteremia outbreaks for clinicians.
Methodology
This meta-analysis was conducted in accordance with the guidelines of PRISMA [7]. All prospective human studies have been approved by the Ethics Committee. The study was performed in accordance with the ethical standards of the 2008 Declaration of Helsinki. In addition, all participants gave informed consent prior to their participation in the study.
Search strategy and study selection
We made a search through Embase, Pubmed and Cochrane databases, screening for the reports concerning the CRE bacteremia in hospitalized adult patients among the published literature before May 2019. The key search terms “(bacteremia OR bacteremia’s OR hemorrhagic septicemia OR bloodstream infection) AND (Carbapenem-Resistant Enterobacteriaceae OR CRE OR Carbapenemase-Producing Enterobacteriaceae OR KPC OR VIM OR NDM OR OXA-48 OR OXA-181 OR IMP)” were applied to the database: . All the titles and abstracts of the retrieved articles were screened against our inclusion and exclusion criteria. Additional eligible studies were found in the references of the identified studies and a manual search was performed to supplement our study. The inclusion criteria established for these articles were described as follows:
- The articles with original data of adult patients with nosocomial infections confirmed as CRE-BSIs (Nosocomial infections were defined as the infections occurred more than 48 hours after the patients were admitted to hospitals or health-care facilities).
- The included literatures were case-control studies or cohort studies. The control group must be non-CRE-BSIs (including CSE-BSIs) and survivors in the study of risk factors for morbidity and mortality, respectively.
- The outcomes recorded were the definite diagnosis of BSI by blood-culture positivity for a CRE strain in the study of risk factors for morbidity, and the 14-day, 28-day, 30-day or overall mortality in the study of risk factors for mortality. If CRE was isolated from multiple blood cultures, only the first one was included. We excluded literatures which were unpublished or duplicated. National reports, open access materials and abstracts, reviews and studies without enough data were also ruled out. Besides, studies were excluded if they researched on children, or with a simple size of fewer than 10 patients.
Data extraction
Two investigators (ML Wu and QX Lin) rechecked and evaluated the eligibility of included studies independently. The third investigator (SF Huang) joined if they had different opinions. The discrepancies were resolved by consensus. For each included paper, the main characteristics of the study (the title, the first author, the publication year, the study area, the study design, the total number of the subjects) and demographic characteristics and comorbid conditions were sought for our study. Furthermore, the causative pathogen(s), sites of infections, specific antibiotic treatments and outcomes were considered eligible for inclusion as well [8-30].
Quality assessment
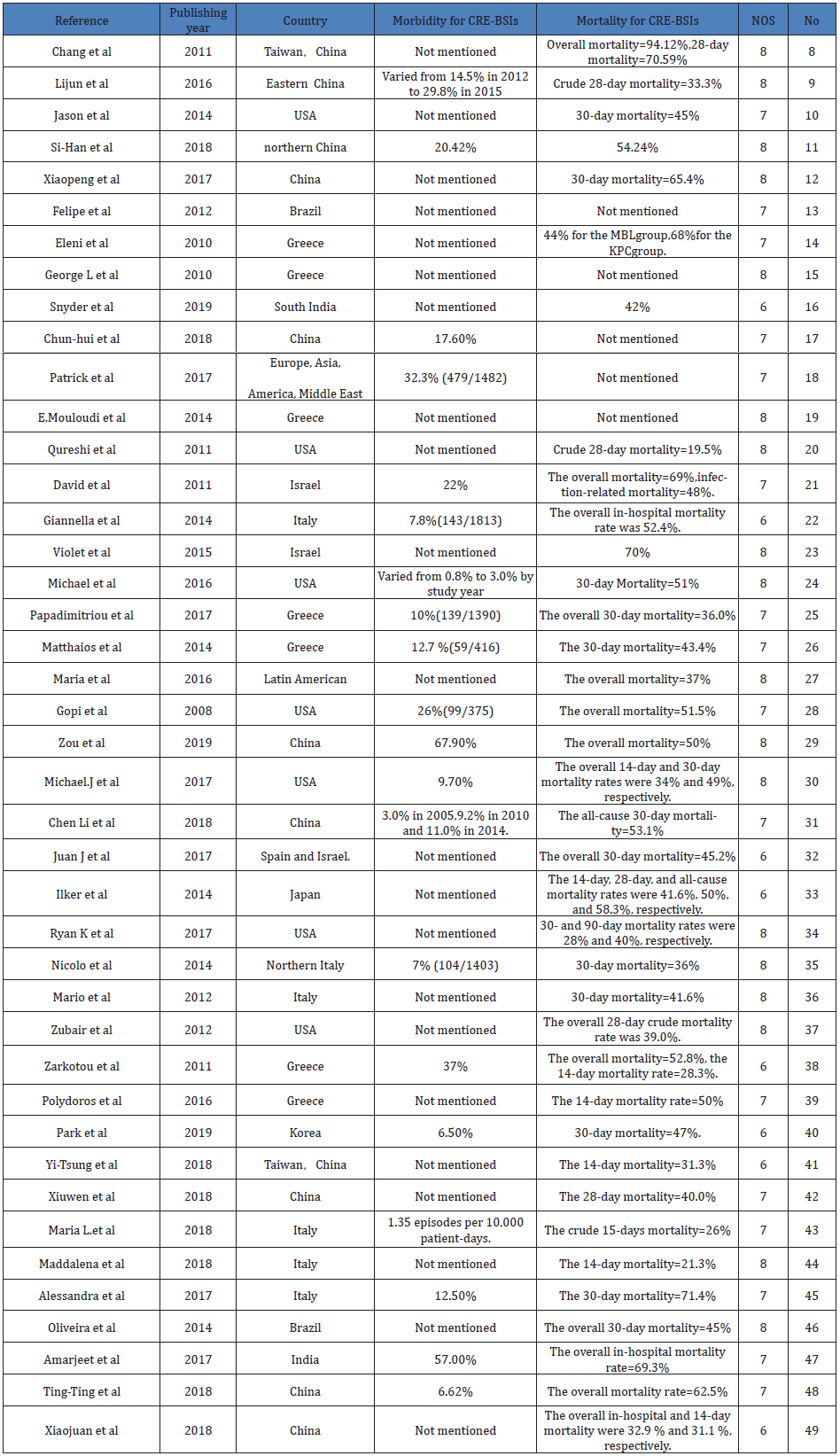
The included papers were all assessed by the Newcastle-Ottawa Quality Assessment Scale (NOS) to reflect the methodological quality. The NOS is a star-based evaluation system, which reflects the observational study quality by ‘Selection’, ‘Comparability’, and ‘Exposure’. Two investigators (ML Wu and QX Huang) separately evaluated each paper. In the end, the mean score was shown as 7 stars in our study (Table1).
Table 1: Summary of studies reporting risk factors for morbidity and mortality related to CRE-BSI.
Statistical analysis
Because of the observed variability between studies, the random effects model was applied as the usual approach to all the variables. For the dichotomous variables, we chose pooled odds ratios (OR) with 95% confidence intervals (CI) to show the combined results. Continuous variables with a normal distribution were expressed as mean ± standard deviation. Considering the presence of heterogeneity, the Q test with a significance level set at P<0.10 was used, and the heterogeneity was quantified with I² statistics (0%-40%, no heterogeneity; 30%-60%, moderate heterogeneity; 50%-90%, substantial heterogeneity; and 75%-100%, considerable heterogeneity). Sensitivity and subgroup analyses were performed to explain the sources of heterogeneity if I²>50%. For the risk factors which included more than five studies, a funnel plot was used to reveal publication biases. The statistical analyses were carried out by the Review Manager 5.3.
Results
Figure 1: Flow diagram of study selection for the systematic review.
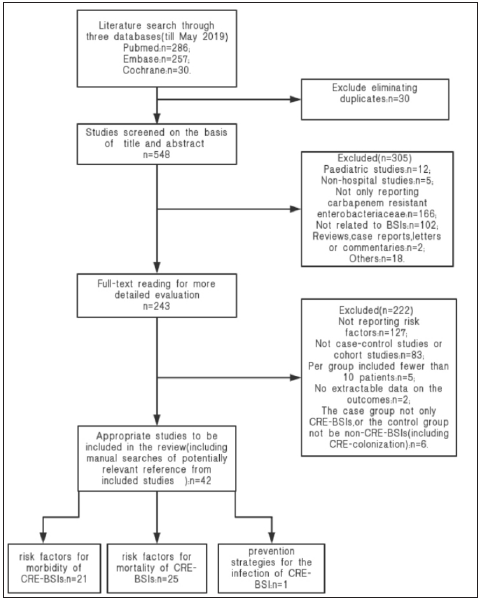
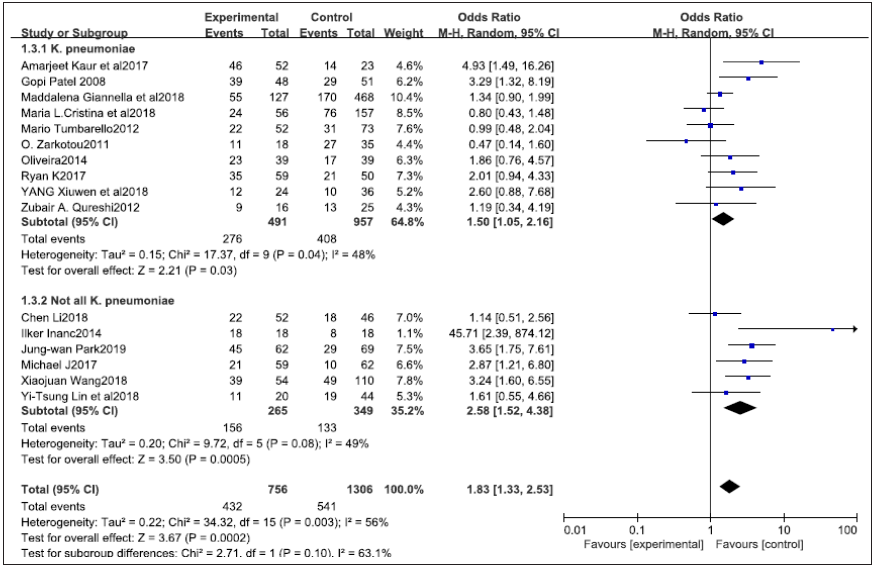
Figure 2: The forest plot of ICU admission as a risk factor for CRE-BSI mortality.
Across the three databases, totally 573 literatures were retrieved out up until May 2019: 286 in Pubmed, 257 in Embase, 30 in Cochrane. A second screening was carried out for the remaining 243 articles based on the full text. And finally, we identified 42 studies [6-12,14-29] to calculate the statistically significant pooled odds ratio, including 22 papers described factors associated with CRE-BSI morbidity and 26 papers with CRE-BSI mortality, as 6 papers were relevant to both morbidity and mortality (Figures 1 & 2). A total of 5774 people was recruited into the meta-analysis of risk factors for morbidity, while 2611 patients were enrolled into the meta-analysis of risk factors for mortality. The size of each study ranged from 14 patients to 1482 patients. In addition, the study period varied widely, ranging from 2004 to 2018. Except for two articles that were written in Chinese, all the other ones were written in English. Since several risk factors were involved in few patients in certain studies, only factors involved in more than three articles were taken into account.
Morbidity for CRE-BSIs
Of the 22 studies related to the risk factors for morbidity, 17 were case-control studies [8-12,15-20,22,24-29], 2 were nested case-control studies [13,14], and 3 were observational cohort study [18,21,23]. Besides Asia, the articles covered North America, Europe, Africa, and Latin America, including Argentina, Colombia, Ecuador, Guatemala, Mexico, Peru, and Venezuela. Except for the 11 studies that didn’t refer to the CRE-BSI morbidity, statistics suggested that the incidence of CRE-BSI ranged from 0.8% to 67.90% during the study period. Of note, an alarming phenomenon is that the morbidity of CRE-BSI has been increasing year by year. 4 out of the 22 studies used a study design involving only the ICU. Among the included studies, the main microorganisms isolated were Klebsiella pneumoniae. As the second most common pathogen responsible for Gram-negative bacteria infection, Klebsiella pneumoniae was reported as the exclusive cause of CRE bacteremia in 11 studies [10,13-15,19-22,25-26,28]. Two studies described the progression to infection of CRE-BSI after colonization. Our meta-analysis regarding CRE-BSI morbidity identified 37 risk factors (OR> 1) and 2 protective factors (OR<1). The risk factors concluded from the multivariable analysis that showed the highest pooled OR for CRE-BSI were previous antibiotic exposure (OR 7.71; 95% CI 2.82-21.08; I-squared=87%), followed by mechanical ventilation (OR 4.54; 95% CI 2.55, 8.08; I-squared=78%) and admission to ICU (OR 4.17; 95% CI 3.02-5.76; I-squared=72%). Compared with patients without urinary catheter or central venous catheter, the patients who had used them before CRE-BSI onset were at a higher risk of infection (OR 3.46; 95% CI 1.89-6.35; I-squared=82%, OR 3.44; 95% CI 2.00-5.92; I-squared=78%, respectively). Additionally, the history of surgery or tracheostomy also showed statistically significant differences in morbidity (OR 1.80; 95% CI 1.26-2.56; I-squared=63%, OR 3.88; 95% CI 1.45-10.40; I-squared=83%, respectively). Furthermore, patients with underlying diseases or conditions, such as renal disease (OR 1.49; 95% CI 1.05-2.11; I-squared=53%), cerebrovascular disease (OR 1.95; 95% CI 1.11-3.44; I-squared=0%), immunosuppression (OR 1.49; 95% CI 1.05-2.11; I-squared=18%), were more likely to develop CRE-BSI. By contrast, empirical antibiotic therapy was classified as a protective factor for CRE-BSI infection (OR 0.06; 95% CI 0.02-0.17; I-squared =80%). Whereas there were several pooled data showing significant differences in morbidity, most of the risk factors showed substantial heterogeneities in our meta-analyses. As a result, the sensitivity analysis and subgroup analysis were made to further clarify those issues. Given that the patients with previous exposures to any antibiotics were more likely to acquire CRE-BSIs, we further calculated the pooled OR for prior antibiotic exposures, and the following categories of antibiotics were found to be significant risk factors: carbapenems (OR 4.18; 95% CI 2.57-6.79; I-squared=77%), cephalosporins (OR 2.15; 95% CI 1.47-3.13; I-squared=30%), colistin (OR 2.81; 95% CI 1.79-4.43]; I-squared=28%), fluoroquinolone (OR 2.52; 95% CI 1.64-3.87; I-squared=59%), aminoglycosides (OR 1.89; 95% CI 1.16-3.09; I-squared=68%), linezolid (OR 3.40; 95% CI 2.06,-5.61; I-squared=52%), β-lactam lactamase inhibitor (OR 1.86; 95% CI 1.22- 2.84; I-squared=63%). Publication bias indicators can be seen for all the above-mentioned antibiotics except for cephalosporins, colistin, and β-lactam lactamase inhibitors Table 2.
Table 2: Results of meta-analyses for the risk factors of CRE-BSI morbidity.
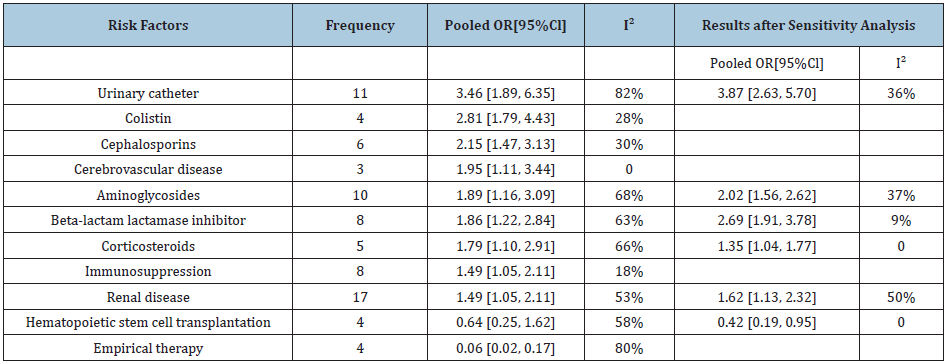
As stated above, the effects of the different variables were reviewed in 20 meta-analyses by performing subgroup analysis if the number of involved studies were more than 5, including the ICU study history, the strain composition (only including K. pneumoniae isolates or not), and the carbapenem resistance mechanisms (only carbapenemase-producing or not). The ICU admission had a great influence on β lactamase inhibitor, as the pooled OR was 1.14 in ICU subgroup (95% CI 0.81-1.60; I-squared =0%) and 2.69 in other subgroups (95% CI 1.91-3.78; I-squared =9%). None of others ultimately revealed a statistically significant difference in the further stratification. However, when compared with the subgroups with the other carbapenem resistance mechanisms, the pooled OR showed dramatic decreases for almost all the potential risk factors in the carbapenem-producing subgroup, apart from immunosuppression, renal disease, fluoroquinolone, central venous catheter or any antibiotic use. Our results were comparable to the other reports. Sensitivity analysis was conducted to investigate the influence of each single study. In total, the heterogeneity of 5 meta-analyses can be explained by the sensitivity analysis. After removing the studies on hematologic malignancies, I² decreased to 0 for both groups, and the pooled OR changed into 1.35 (95%CI 1.04-1.77) and 0.42(95%CI 0.19-0.95) for corticosteroids and hematopoietic stem cell transplant group, respectively. The I² decreased to 36% and the pooled OR changed into 3.87 (95%CI 2.63, 5.70) when the study for CRE rectal carriers was removed in the urinary catheter group. On the other hand, the study with the patients infected by the ESBLs-producers as the control was considered as the source of heterogeneity for renal disease as I² decreased to 50% while the pooled OR turned into 1.62 (95%CI 1.13-2.32).The heterogeneity we mentioned above can be explained by the particularity of research objects involved in different studies. As for the risk factor aminoglycosides, the heterogeneity was caused by the studies conducted in ICU (pooled OR 2.02; 95% CI 1.56-2.62; I-squared=37%). The data of other risk factors the included studies ever reported, including age, gender, any antibiotic use, cardiovascular disease, heart failure, chronic pulmonary disease, diabetes, receiving enteric tube feeds, glycopeptides, liver disease, malignancy, metronidazole, penicillins, quinolones, tigecycline, parenteral nutrition, trauma, peptic ulcer disease, solid tumor, HIV, and chemotherapy, were also extracted,. However, none of those data related to risk factors showed correlations with the development of CRE-BSI, besides, the obvious heterogeneity can’t be explained as the few studies involved. What’s more, we also gathered statistics related to duration of hospitalization before CRE-BSI onset in 8 studies [8,11-15,24,29], however, the pooled OR can’t be calculated because of the inconformity of the variables. Except for one study [13], all included papers reflected a longer duration of hospitalization in the infected group, which can be easily understood because the longer the patients are going to stay in hospitals, the more likely they are going to be infected by CRE [30-41].
Mortality for CRE-BSIs
The next section showed the characteristics of the 26 studies on mortality. Out of the 26 studies, 5 were conducted in USA, 6 in China, 4 in Greece, 5 in Italy, 2 in Latin America, 1 in Spain & Israel, Japan, Korea and India, respectively [42-50]. Like the case patients in the term of morbidity, the most common organism isolated from the dead cases was Klebsiella pneumoniae. The calculated 30-day mortality varied from 28% to 71.4% in 11 studies [24,26,30-32,34-36,40,45,46]. The 14-day mortality varied from 21.3% to 50% in 7 studies [30,33,38,39,41,44,49] and the 28-day mortality varied from 39% to 50% [33,37,42]. 9 studies also reported the overall mortality, ranging from 32.9% to 69.3% [25,27-29,33,38,47-49]. Table 3 summarized the results of our meta-analysis on mortality of CRE-BSI. We calculated the pooled OR related to 38 risk factors of mortality, each one was extracted in more than three articles respectively. Consistent with other studies, the factors demonstrated to be significantly associated with death included malignancy (OR 1.70; 95% CI 1.18-2.43; I-squared=0%), chronic renal disease (OR 1.59; 95% CI 1.21-2.08; I-squared =0%), hemodialysis (OR 1.74; 95% CI 1.11-2.73; I-squared=0%), septic shock (OR 6.71; 95% CI 4.56-9.88; I-squared=39%), monotherapy (OR 1.62; 95% CI 1.00-2.64; I-squared=45%), not appropriate empirical treatment (OR 0.54; 95% CI 0.29-1.00; I-squared=48%), heart disease (OR 2.30; 95% CI 1.08-4.88; I-squared=26%), liver disease (OR 1.59; 95% CI 1.12-2.24; I-squared=0%), mechanical ventilation (OR 2.02; 95% CI 1.50-2.71; I-squared=1%), indwelling urinary catheter (OR 2.02; 95% CI 1.50-2.71; I-squared=1%), neutropenia (OR 1.64; 95% CI 1.11-2.43; I-squared=0%), prior use of antibiotics (OR 1.09; 95% CI 1.01-1.18; I-squared =0%), previous carbapenem exposures (OR 2.22; 95% CI 1.23-4.03; I-squared =47%), and corticosteroids use (OR 1.84; 95% CI 1.03-3.29; I-squared =42%). The subgroup analysis and sensitivity analysis were performed to reveal the heterogeneity sources. As previously documented, 3 categories of the subgroup were done for each risk factors. For the studies related to patients in ICU, processed result showed a significant difference between the K. pneumoniae subgroup and the other subgroups. Compared with the other subgroups, the pooled OR reduced if the pathogen only included K. pneumoniae. Otherwise, none of the meta-analysis revealed statistical difference in subgroup analysis. The pooled OR changed to 0.5 (95% CI 0.32-0.80) after removing the ICU study for the primary bacteremia group, with the decreasing I-squared changing from 68% to 33%. What’s more, there is no evidence to prove the effect of age, colistin, APACHE2 score for the mortality. Both subgroup analysis and sensitivity analysis can’t explain the heterogeneity. There are other risk factors which were mentioned in several studies showing a positive correlation with mortality, whereas our results are not in alignment with them. In our meta-analysis, no differences among gender, chronic pneumopathy, central venous catheter, diabetes, hematologic malignancy, HIV, polymicrobial BSI, solid tumor, surgery, transplant recipients, tracheotomy, immunosuppressive therapy, length of stay in hospital, and the prior exposure to tigecycline, amikacin, or the resistance of colistin or tigecycline were detected. Besides colistin and corticosteroids, the involved studies reported other risk factors for mortality, for example, a study performed in intensive care units about KPC-producing Klebsiella pneumoniae bloodstream drew a conclusion that gentamicin resistance, tigecycline resistance, and colistin resistance may lead to a poor prognosis [13]. The combination therapy using “tigecycline + colistin + meropenem” might result in lower mortality than other combinations in another study [37]. Moreover, while ceftazidime-avibactam was considered as a protective factor for mortality by Ryan K et al. [34], higher Pitt bacteremia score or APACHE2 score were recommended as predictors for mortality of CRE-BSIs.
Table 3: Results of meta-analyses for the risk factors of CRE-BSI mortality.

Analysis of publication bias
Funnel plot regression was used to evaluate risk factors for more than five studies. None of the publication biases existed.
Infection prevention strategies
There are two protective factors for CRE-BSI mortality, appropriate empirical therapy (n=4) and hematopoietic stem cell transplantation (n=3). One systematic review related to CRE acquisition also revealed that the use of barrier and/or contact precautions, patient cohorting and active surveillance were the most successful intervention strategies [50]. For the mortality of CRE-BSI, the protective factors involved the use of ceftazidime-avibactam, lower Pitt bacteremia score or APACHE2 score were also relevant. Likewise, different score or predictive model was used to predict the mortality as Violet reported [23,38,51].
Discussion
Many studies investigating the risk factors regarding to the morbidity and mortality of CRE-BSIs have been published in the past decade, while few systematic reviews and meta-analysis were performed recently. We made this meta-analysis to further elucidate the risk factors for both the morbidity and mortality of CRE-BSIs among worldwide hospitalized patients. From a clinical and epidemiological perspective, identification of factors in patients with CRE-BSI is very important for both the understanding of the determinants of the dissemination of these bacteria and potentially guiding empirical antimicrobial choices. As stated above, we finally defined 9 risk factors of CRE-BSIs in our review, which were shown in order of those with the highest to the lowest pooled ORs in Table 2. Comparable to the previous reports, urinary catheter generated the highest pooled estimate, followed using colistin. We therefore deemed that reducing invasive procedure or making it more standardly are closely linked to a reduced risk of infection. In contrast, like invasive operation, patients with central venous catheter or history of surgery presented substantial or moderate heterogeneities. Therefore, additional analyses including subgroup analysis and sensitivity analysis were performed. ICU admission and CRE rectal carriers make a signficiant difference in aminoglycosides and urinary catheter group, respectively. For those neutropenic patients, hematopoietic stem cell transplant is a protective factor. Even though the publication bias led to substantial heterogeneity, empirical therapy was recommended as a kind of prevention strategies in all the incorporated studies. According to our pooled ORs, patients with a previous history of antibiotic use, such as colistin, cephalosporins and aminoglycosides, were more likely to infect CRE-BSIs, thus previous antibiotic use was proved to be a risk factor for morbidity. Additionally, other antibiotics attributing to CRE-BSI, such as linezolid, tigecyclines, corticosteroids, and glycolpeptides, which are frequently used for patients with CRE, have also been revealed in the included studies. As previously documented [52], this can be explained by that plasmids responsible for carbapenem resistance often carry additional genes conferring resistance to other antibiotics. The mortality for CRE-BSI reviewed in our paper was consistent with other studies. Totally 16 risk factors were declared in the meta-analysis. We found that a higher risk of death was associated with underlying diseases or conditions, such as malignancy, chronic renal disease, hemodialysis, neutropenia, heart disease, liver disease, and septic shock. Several other studies referring to risk factors accounted Pitt bacteremia score, APACHE2 score to describe the importance of underlying disease, yet because of the inconsistency of the statistics, the pooled ORs can’t be rule out. Additionally, an alarming phenomenon was that both morbidity and mortality could be impacted by invasive procedures such as mechanical ventilation and indwelling urinary catheter. It can be easily understood as a result of the more opportunities of invading blood by CRE. Controversial with other studies, ICU admission was defined as a risk factor for mortality, and the pooled OR reduced if K. pneumoniae was included as the only pathogen . We speculated that the phenomenon was caused by the more serious illness and the more treatments patients in ICU received. Prior use of antibiotics, such as carbapenem, and corticosteroids use also led to poor outcomes, suggesting the necessity of standardizing antibiotic and steroids managements. Tigecycline with colistin, one carbapenem with Fosfomycin or colistin or aminoglycoside have been reported as the most effective combinative treatments to serious infections of CRE [53]. The antibiotic resistances, such as gentamicin resistance, tigecycline resistance, and colistin resistance were considered to have led to a terrible outcome in several studies [54]. Several studies reported successful containment of Carbapenemase producers by implementing a bundle of infection control measures, among which the most important were active surveillance cultures and separation of carriers. Our study also highlighted the effects of empirical therapy and hematopoietic stem cell transplant. Besides appropriate treatment for primary bacteremia, developing a scoring standard to prevent death of CRE-BSI was recommended. These results must be interpreted with caution in view of certain limitations. The first is the paucity of the included studies and the inconsistency of the statistics forced us to present patients together, ignoring the race, different genotypes of the pathogen, and the different geographic locations. Another important issue is that our results were combined using a random effects model in order to take account of the differences expected between studies. However, it would reduce the efficiency of studies with large sample, causing the pooled OR not to be able to reflect the true contribution. Moreover, specific conclusions were drawn from few studies with a small number of patients, and thus randomized controlled trials with large sample sizes are needed. Finally, there are some considerable heterogeneities among meta-analyses that we can’t eliminate by performing the sensitivity analyses and subgroup analyses, although a lot of heterogeneities have been reported in several studies related to risk factors. Briefly, this systematic review and meta-analysis highlights the emergency of the global prevalence for the hospitalized patients with CRE-BSIs, suggests the risk factors of the morbidity and mortality for CRE-BSIs, reflects the importance of standard invasive procedure, empirical antibiotic therapy, rational antibiotic use in preventing CRE-BSIs hospital outbreaks for clinicians. What’s more, either severity score upon infection onset can be used to predicted mortality.
References
- Laupland KB, Church DL (2014) Population based epidemiology and microbiology of community onset bloodstream infections. Clinical Microbiology Reviews 27(4): 647-664.
- Munoz Price LS, Poirel L, Bonomo RA, Schwaber MJ, Daikos GL, et al. (2013) Clinical epidemiology of the global expansion of Klebsiella pneumoniae Lancet Infect Dis 13(9): 785-796.
- Jacob JT, Klein E, Laxminarayan R, Beldavs Z, Lynfield R, et al. (2013) Vital Signs: Carbapenem resistant enterobacteriaceae. Morbidity and mortality weekly report 62(9): 165-169.
- Hussein K, Raz Pasteur A, Finkelstein R, Neuberger A, Shachor Meyouhas Y, et al. (2013) Impact of carbapenem resistance on the outcome of patients hospital acquired bacteraemia caused by Klebsiella pneumoniae. Journal of Hospital Infection 83(4): 307-313.
- Meatherall BL, Gregson D, Ross T, Pitout JD, Laupland KB (2009) Incidence, risk factors, and outcomes of Klebsiella pneumoniae Am J Med 122(9): 866-873.
- Falagas ME, Tansarli GS, Karageorgopoulos DE, Vardakas KZ (2014) Deaths attributable to carbapenem resistant enterobacteriaceae Emerging infectious diseases 20(7): 1170-1175.
- Moher D, Liberati A, Tetzlaff J, Altman DG, PRISMA Group. Preferred reporting items for systematic reviews and meta analyses: the PRISMA statement. PLoS Med 6(7): e1000097.
- Chang HJ, Hsu PC, Yang CC, Kuo AJ, Chia JH, et al. (2011) Risk factors and outcomes of carbapenem nonsusceptible Escherichia coli bacteremia: a matched case-control study. Journal of Microbiology, Immunology and Infection 44(2): 125-130.
- Tian L, Tan R, Chen Y, Sun J, Liu J, et al. (2016) Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control 5: 48.
- Gallagher JC, Kuriakose S, Haynes K, Axelrod P (2014) Case control study of patients with carbapenem resistant and third-generation cephalosporin resistant Klebsiella pneumoniae bloodstream infections. Antimicrobial Agents & Chemotherapy 58(10): 5732-5735.
- Zheng SH, Cao SJ, Xu H (2018) Risk factors, outcomes and genotypes of carbapenem-non-susceptible Klebsiella pneumoniae bloodstream infection: a three-year retrospective study in a large tertiary hospital in Northern China. Infectious Diseases pp. 1-9.
- Xiaopeng L, Huan Y (2017) Clinical and mortality risk factors in bloodstream infections with carbapenem-resistant enterobacteriaceae. Canadian Journal of Infectious Diseases and Medical Microbiology pp. 1-5.
- Tuon FF, Rocha JL, Toledo P (2012) Risk factors for KPC-producing Klebsiella pneumoniae Braz J Infect Dis 16(5): 416-419.
- Mouloudi E, Protonotariou E, Zagorianou A (2010) Bloodstream infections caused by metallo β lactamase/klebsiella pneumoniae carbapenemase-producing pneumoniae among intensive care unit patients in greece: risk factors for infection and impact of type of resistance on outcomes. Infection Control and Hospital Epidemiology 31(12): 1250-1256.
- Daikos GL, Vryonis E, Psichogiou M (2010) Risk factors for bloodstream infection with Klebsiella pneumoniae producing VIM-1 metallo-beta-lactamase. J Antimicrob Chemother 65(4): 784-788.
- Snyder BM, Montague BT, Anandan S (2019) Risk factors and epidemiologic predictors of blood stream infections with New Delhi Metallo-β-lactamase (NDM-1) producing Enterobacteriaceae. Epidemiol Infect 147: e137.
- Xu Chunhui, Su Yang, Lyu Yanxia (2018) Perianal swabs surveillance cultures of Carbapenem-resistant Enterobacteriaceae(CRE) can be hints for CRE bloodstream infection in patients with hematological diseases. Chinese Journal of Hematology 39(12): 1021-1025.
- Patrick NA, Harris MDP, Belén GG (2017) Geographical variation in therapy for bloodstream infections due to multidrug-resistant enterobacteriaceae: a post hoc analysis of the INCREMENT study. Int J Antimicrob Agents 50(5): 664-672.
- Mouloudi E, Massa E, Papadopoulos S (2014) Bloodstream infections caused by carbapenemase-producing klebsiella pneumoniae among intensive care unit patients after orthotopic liver transplantation: risk factors for infection and impact of resistance on outcomes. Transplantation Proceedings 46(9): 3216-3218.
- Qureshi Z, Paterson D, Peleg A (2012) Clinical characteristics of bacteraemia caused by extended-spectrum beta-lactamase-producing Enterobacteriaceae in the era of CTX-M-type and KPC-type beta-lactamases. Clin Microbiol Infect 18(9):887-893.
- Ben D, Kordevani R, Keller N (2012) Outcome of carbapenem resistant Klebsiella pneumoniae bloodstream infections. Clin Microbiol Infect 18(1): 54-60.
- Giannella M, Trecarichi EM, Rosa FGD (2014) Risk factors for carbapenem-resistant Klebsiella pneumoniae bloodstream infection among rectal carriers: a prospective observational multicentre study. Clin Microbiol Infect 20(12): 1357-1362.
- Violet L, Emily TM, Ruthy TJ (2015) Simple bedside score to optimize the time and the decision to initiate appropriate therapy for carbapenem-resistant Enterobacteriaceae. Ann Clin Microbiol Antimicrob 14(1): 31.
- Satlin MJ, Cohen N, Ma KC (2016) Bacteremia due to carbapenem-resistant enterobacteriaceae in neutropenic patients with hematologic malignancies. J Infect 73(4): 336-345.
- Papadimitriou OM, Fligou F, Bartzavali C (2017) Carbapenemase-producing Klebsiella pneumoniae bloodstream infection in critically ill patients: risk factors and predictors of mortality. Eur J Clin Microbiol Infect Dis 36(7): 1125-1131.
- Papadimitriou OM, Marangos M, Christofidou M (2014) Risk factors for infection and predictors of mortality among patients with KPC-producing Klebsiella pneumoniae bloodstream infections in the intensive care unit. Scandinavian Journal of Infectious Diseases 46(9): 642-648.
- Virginia VM, Pallares CJ, Escandón VK (2016) Characterization and clinical impact of bloodstream infection caused by carbapenemase-producing enterobacteriaceae in seven Latin American countries. PLOS ONE 11(4): e0154092.
- Patel G, Huprikar S, Factor SH (2008) Outcomes of Carbapene in resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infection Control and Hospital Epidemiology 29(12): 1099-1106.
- Hua Z, Qiuxia L, Senjie X, Menglu W (2019) CP-CRE/non-CP-CRE stratification and CRE resistance mechanism determination might be helpful for better management of CRE bacteremia by aztreonam-avibactam. Infect Drug Resist 12: 3017-3027.
- Satlin MJ, Chen L, Patel G (2017) Multicenter clinical and molecular epidemiological analysis of bacteremia due to carbapenem-resistant enterobacteriaceae (CRE) in the CRE epicenter of the United States. Antimicrobial Agents & Chemotherapy 61(4): 02349-16.
- Chen L, Yi L, Zhichang Z (2018) Treatment options and clinical outcomes for carbapenem-resistant Enterobacteriaceae bloodstream infection in a Chinese university hospital. Journal of Infection and Public Health 12(1): 26-31.
- Castón JJ, Lacort I, Martíndávila P (2017) Clinical efficacy of ceftazidime/avibactam versus other active agents for the treatment of bacteremia due to carbapenemase-producing enterobacteriaceae in hematologic patients. Int J Infect Dis 59: 118-123.
- Balkan II, Aygün G, Aydın S (2014) Blood stream infections due to OXA-48-like carbapenemase-producing Enterobacteriaceae: treatment and survival. Int J Infect Dis 26: 51-56.
- Shields RK, Nguyen MH, Chen L (2017) Ceftazidime avibactam is superior to other treatment regimens against carbapenem-resistant klebsiella pneumoniae Antimicrob Agents Chemother 61(8): 00883-00917.
- Girometti N, Lewis RE, Giannella M (2014) Klebsiella pneumoniae bloodstream infection: epidemiology and impact of inappropriate empirical therapy. Medicine 93(17): 298-309.
- Tumbarello M, Viale P, Viscoli C (2012) Predictors of mortality in bloodstream infections caused by klebsiella pneumoniae carbapenemase-producing pneumoniae: importance of combination therapy. Clin Infect Dis 55(7): 943-950.
- Qureshi ZA, Paterson DL, Potoski BA (2012) Treatment outcome of bacteremia due to kpc-producing klebsiella pneumoniae: superiority of combination antimicrobial regimens. Antimicrobial Agents and Chemotherapy 56(4): 2108-2113.
- Zarkotou O, Pournaras S, Tselioti P (2011) Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 17(12): 1798-1803.
- Tofas P, Skiada A, Angelopoulou M (2016) Carbapenemase-producing Klebsiella pneumoniae bloodstream infections in neutropenic patients with haematological malignancies or aplastic anaemia: analysis of 50 cases. International Journal of Antimicrobial Agents 47(4): 335-339.
- Park JW, Lee H, Park SY, Kim TH (2019) Epidemiological, clinical, and microbiological characteristics of carbapenemase-producing Enterobacteriaceae bloodstream infection in the Republic of Korea. Antimicrob Resist Infect Control 8: 48.
- Lin YT, Su CF, Chuang C (2018) Appropriate treatment for bloodstream infections due to carbapenem-resistant klebsiella pneumoniae and escherichia coli: a nationwide multicenter study in Taiwan. Open Forum Infect Dis 6(2): 336.
- Xiuwen Y, Junchang C, Jin Z (2018) Clinical characteristics of carbapenem-resistant Klebsiella pneumoniae bloodstream infection and the risk factors of mortality. Chinese Journal of Infection and Chemotherapy 18(2): 142-149.
- Cristina ML, Alicino C, Sartini M, Faccio V, Spagnolo AM, et al. (2017) Epidemiology, management, and outcome of carbapenem-resistant, Klebsiella pneumoniae, bloodstream infections in hospitals within the same endemic metropolitan area. Journal of Infection and Public Health 11(2): 171-177.
- Giannella M, Trecarichi EM, Giacobbe DR (2018) Effect of combination therapy containing a high dose carbapenem on mortality in patients with carbapenem-resistant, klebsiella pneumoniae, bloodstream infection. Int J Antimicrob Agents 51(2): 244-248.
- Micozzi A, Gentile G, Minotti C (2017) Carbapenem-resistant Klebsiella pneumoniae in high-risk haematological patients: factors favouring spread, risk factors and outcome of carbapenem-resistant Klebsiella pneumoniae BMC Infectious Diseases 17(1): 203.
- Oliveira MS, Assis DB, Freire MP, Boas Prado GV, Machado AS, et al. (2015) Treatment of KPC-producing Enterobacteriaceae: suboptimal efficacy of polymyxins. Clinical Microbiology and Infection 21(2): 179.e1-179.e7.
- Kaur A, Gandra S, Gupta P (2017) Clinical outcome of dual colistin- and carbapenem-resistant, Klebsiella pneumoniae, bloodstream infections: A single-center retrospective study of 75 cases in India. Am J Infect Control 45(11): 1289-1291.
- Geng, Ting, Xu (2018) High-dose tigecycline for the treatment of nosocomial carbapenem-resistant Klebsiella pneumoniae bloodstream infections: A retrospective cohort study. Medicine 97(8): e9961.
- Xiaojuan W, Qi W, Bin C, Shijun S (2018) Retrospective observational study from a chinese network of the impact of combination therapy versus monotherapy on mortality from carbapenem-resistant enterobacteriaceae Antimicrobial Agents and Chemotherapy Dec 63(1): e01511-18.
- Van Loon K, Voor IAF, Vos MC (2017) A systematic review and meta-analyses of the clinical epidemiology of carbapenem-resistant Antimicrob Agents Chemother 62(1): e01730-17.
- Gutiérrez G, Belén, Salamanca E, De Cueto M, Lowman W, et al. (2016) A predictive model of mortality in patients with bloodstream infections due to carbapenemase-producing enterobacteriaceae. Mayo Clinic Proceedings 91(10): 1362-1371.
- Meatherall BL, Gregson D, Ross T (2009) Incidence, risk factors, and outcomes of Klebsiella pneumoniae Am J Med 122(9): 866-873.
- Falagas ME, Karageorgopoulos DE, Nordmann P (2011) Therapeutic options for infections with Enterobacteriaceae producing carbapenem hydrolyzing enzymes. Future Microbiol 6(6): 653-666.
- Zarkotou O, Pournaras S, Voulgari E (2010) Risk factors and outcomes associated with acquisition of colistin-resistant KPC-producing Klebsiella pneumoniae: a matched case-control study. J Clin Microbiol 48(6): 2271-2274.
© 2020 Huang Shifeng. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






