- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Comparison of Fulminantly Fatal Bacteremias Due to Extensively-Drug Resistant Klebsiella Pneumoniae and Acinetobacter Baumannii in a Multidisciplinary Intensive Care Unit During an 8- Years’ Period
Maria Katsiari1*, Angeliki Mavroidi2, Evangelia D Platsouka2 and Charikleia Nikolaou1
1Intensive Care Unit, Konstantopouleio Patission General Hospital, Greece
22Department of Microbiology, Konstantopouleio Patission General Hospital, Greece
*Corresponding author: Katsiari Maria, Intensive Care Unit, Konstantopouleio Patission General Hospital, Greece
Submission: January 17, 2020; Published: February 5, 2020

ISSN 2578-0190 Volume3 issues3
Abstract
Background: Infections due to extensively drug-resistant gram-negative bacteria entail limited treatment options and high mortality and pose an important public health problem. Severe infection causes multiple organ dysfunction through complex mechanisms, which involve either the host, or the pathogenicity of the microorganism. The aims of the present study were the determination of potential risk factors of a fulminant fatal course of bacteremia caused by extensively drug-resistant Klebsiella pneumoniae(XDR- Kp) and the comparison of fulminantly fatal bacteremias due to XDR-Kp versus XDR Acinetobacter baumannii (XDR-Ab) strains within a 8-years’ period.
Methods: Patients with monomicrobial XDR-Kp (n=60) or XDR-Ab (n=87) bacteremias were divided into three groups, according to ICU outcome (survival, early or late death). Τhe clinical characteristics of patients along with pathogen and treatment-related factors were compared by ANOVA statistics and multinomial logistic regression.
Results: Patients with unfavorable outcome revealed higher disease severity at ICU admission and on bacteremia day. Administration of corticosteroids or renal replacement treatment on infection day was significantly higher in the fulminantly fatal group. Pathogens’ resistance pattern and appropriate treatment did not affect outcome. Fulminant bacteremias due to CR-Kp emerged significantly later during ICU hospitalization compared to XDR-Aba. Pathogens’ resistance pattern, regarding colistin and tigecycline, differed between XDR-Kp and XDR-Ab strains. Conclusions: Higher disease severity and administration of corticosteroids and renal replacement therapy posed patients at higher risk for fulminantly fatal course of CR-Kp bacteremia. Receipt of appropriate treatment did not affect outcome.
Keywords: Extensively-drug resistant Klebsiella pneumoniae; Extensively-drug resistant Acinetobacter baumannii; Bacteremia; Risk factors; Fulminant sepsis; ICU mortality
Introduction
Klebsiella pneumoniae (Kp) is ubiquitous in nature and causes various human infections, including invasive infections in healthcare settings [1]. It has been reported as the second most common pathogen in Enterobacteriaceae bloodstream infections (BSIs), following Escherichia coli. Several factors have been associated with mortality related to Kp bloodstream infections, such as a high Sepsis Organ Failure Assessment (SOFA) score and Acute Physiology Chronic Health Evaluation (APACHE II) score, the lung as probable source of infection, septic shock, inadequate initial antimicrobial therapy and the dissemination of carbapenem-resistant Klebsiella pneumoniae (CR-Kp) strains [2,3]. Antimicrobial resistance and virulence are generally considered as significant factors in the pathogenicity of Kp. A recent meta-analysis of mortality of patients infected with CR-Kp suggested that these patients had higher mortality than those infected with carbapenem-susceptible Kp, especially in association with bloodstream infections and Intensive Care Unit (ICU) admission [4]. Infections due to extensively drug-resistant Klebsiella pneumoniae (XDR-Kp), such as pneumonia, urinary tract infections and bloodstream infections [1] entail limited treatment options and pose an important public health problem. Nevertheless, antimicrobial resistance might not be the major factor responsible for an unfavorable outcome. Severe infection causes multiple organ dysfunction through complex mechanisms, which involve either the host, such as an excessive inflammatory response and immune dysfunction, or the pathogenicity of the microorganism. Differences in Kp infection based on patients’ outcome may modify and optimize the treatment approaches of these patients in clinical practice. In the present study, we investigated BSIs caused by XDR-Kp in a multidisciplinary ICU during the past 8 years. We compared various clinical characteristics of patients along with pathogen and treatment-related factors among patients who survived and those with unfavorable outcome (early or late), in order to determine potential predictors of a fulminant fatal course of bacteremia. In a previous work [5], we have shown that patients suffering from a fulminant course of XDR-Aba bacteraemia showed significantly higher rates of chronic renal failure and multiple organ dysfunction. Resistance patterns of XDR-Aba isolates and receipt of appropriate treatment did not affect outcomes. Since these two pathogens are predominant in our ICU, we also sought to compare the characteristics of patients with fulminantly fatal sepsis with XDR Acinetobacter baumannii (XDR-Ab) with those with XDR-KP fulminant course.
Materials and Methods
Study setting, design and definitions: This retrospective, observational study was conducted at the nine-bed multidisciplinary ICU of Konstantopouleio-Patission General Hospital (Athens, Greece), which is a 280-bed tertiary-care hospital. During the study period (January 1st, 2011 to December 31st, 2018) all patients with monomicrobial growth of CR-Kp (n=60) or monomicrobial growth of CR- Ab (n=87) in blood cultures were included in the study. XDR-Kp isolates and XDR-Ab were considered when they were non-susceptible to at least one agent in all but two or fewer antimicrobial categories. All CR-Kp and CR-Ab were categorized as XDR in the present study. Pan-drug-resistant (PDR) Kp or Ab were considered the non-susceptible isolates to all antimicrobial agents in all antimicrobial categories [6]. Data were collected anonymously for each patient and included: demographic characteristics, co-morbidities, reason for ICU admission, disease severity at ICU admission as determined by APACHE II score, organ dysfunction at ICU admission and at the time of bacteremia as determined by SOFA score, ICU length of stay (LOS) and outcome. At the onset of bacteremia we recorded length of prior hospitalization (in a general ward and ICU), previous or current receipt of carbapenems, and/or colistin, and/or tigecycline, and/or aminoglycoside, administration of glucocorticosteroids and application of central venous catheter (CVC), mechanical ventilation and renal replacement therapy. Klebsiella pneumoniae bacteremia was diagnosed if an isolate of Kp from one or more blood cultures was accompanied by septic status of the patient, as defined by the Surviving Sepsis Campaign Definitions of 2016 [7]. The date of the first positive blood culture was considered the onset of bacteremia. In cases that a patient had more than one bacteremic episode, only the first episode was included in the analysis. The same criteria were applied for Acinetobacter baumannii bacteremia determination. Definitions of bacteremia were described as follows: 1) Primary bacteremia comprises a) bloodstream infection of unknown origin in patients without identifiable focus for the infection and b) intravascular catheter-related bloodstream infection. 2) Secondary bacteremia comprises bloodstream infection related to an infection at another body site (such as pneumonia, bone or joint infection, meningitis, etc). To analyze the impact of antimicrobial resistance on clinical outcome, the appropriateness of antimicrobial therapy was assessed. An appropriate antimicrobial therapy was defined as the administration of at least one antimicrobial agent to which pathogen was susceptible in vitro within 48 hours after bacteremia onset, with an approved route and dosage; otherwise, the antimicrobial therapy was considered inappropriate [8]. Empiric treatment regimens were selected at the discretion of the attending physician, according to Surviving Sepsis Campaign guidelines regarding empiric antimicrobial therapy in patients with sepsis and septic shock and the susceptibility patterns of Kp isolates in our hospital. Sixty patients with XDR-Kp bacteremia were divided into three groups, according to their ICU outcome. Group A (n=25) consisted of patients who survived, whereas patients that died early (within 48h) or later (>48h) after the onset of bacteremia were allocated to Group B (n=13) and Group C (n=22), respectively. Fulminant sepsis applies to Group B patients, dying within the first 48 h after the onset of bacteremia. Eighty-seven patients with XDR-Ab bacteremia were also divided into three corresponding outcome groups. The 14 patients that comprised the group with XDR-Ab fulminant sepsis were compared with the XDR-Kp fulminant sepsis group (Group B).
Antimicrobial susceptibility testing and molecular identification of Klebsiella pneumoniae and Acinetobacter baumannii bloodstream isolates: Identification of the isolates and antimicrobial susceptibility testing were performed by the Micro Scan® (Siemens Healthcare, PA, USA) and the VITEK 2 (BioMérieux) automated systems, according to the Clinical and Laboratory Standards Institute (CLSI, 2018) interpretive standards [9]. The minimum inhibitory concentrations (MICs) of imipenem, meropenem, colistin, tigecycline and gentamicin were additionally determined using the MIC Test Strips (Liofilchem S.R.L) on Mueller Hinton (MH) Agar plates. For colistin-resistant or isolates with discrepant MIC results, the colistin MICs were confirmed by the SensiTest Colistin (Liofilchem S.R.L, Italy) and the MIC-Strip Colistin (Merlin, Diagnostika, GmbH, Germany) broth microdilution methods, according to the instruction of the manufacturers. Klebsiella pneumoniae and Acinetobacter baumannii isolates with MICs of colistin and tigecycline >2mg/L were categorized as resistant, according to the European Committee on Antimicrobial Susceptibility Testing (EUCAST) interpretive criteria (http://www.eucast.org). For the phenotypic detection of carbapenemase production and the differentiation of KPC and MBL enzymes, the combined-disc tests were used as described previously [10]. The production of KPC, OXA-48, VIM and NDM carbapenemases was determined by the immunochromatographic tests KPC K-SeT, OXA-48 K-SeT and RESIST-4 O.K.N.V., CORIS, Bio Concept, Belgium [11,12]. DNA extraction was carried out using the QIAcube system (Qiagen, Düsseldof, Germany), according to the instructions of the manufacturer. Carbapenemase-encoding genes (blaKPC, blaOXA-48, blaVIM and blaNDM) [13,14] were detected by PCR using specific primers, as described previously.
Statistical analysis
Continuous variables were expressed as mean ± standard deviation (SD) or median (interquantile range). Continuous variables were compared with oneway analysis of variance (ANOVA). When analysis of variance revealed a significant difference, all pairwise multiple comparison procedures were executed with Holm-Sidak method. Categorical variables were evaluated with the Chi-square test or Fischer’s exact test. The demographic and clinical characteristics of the patients affected by XDR Kp were assessed for identifying risk factors of the outcome for fulminant sepsis (Group B) as a reference category v.s. late sepsis (Group C) patients and survivors (Group A) by multinomial logistic regression in XLSTAT 2019.3.2.61904. Binary logistic regression was performed for identifying risk factors for XDR Kp vs XDR-Ab. A two-tailed p-value <0.05 was considered statistically significant.
Results
The anthropometric and clinical characteristics of study population affected by XDR-Kp during the 8-year’s study period are presented in Table 1. Crude mortality was 58.3% (35/60) whereas attributed mortality accounted for 35% (21/60). After patients’ allocation in three groups according to their ICU outcome, the sex distribution and mean age were similar between the groups. Patients that died both early or later revealed higher disease severity and organ dysfunction at ICU admission, as determined by APACHE II and SOFA, respectively. Cause of admission was mainly medical in all groups. Prior length of hospitalization did not differ among the groups. Fulminant fatal bacteremias emerged later during ICU hospitalization, albeit the difference did not reach statistical significance.
Risk factors for fulminant sepsis
Host-related risk factors: Regarding patients’ comorbidities, the prevalence of diabetes mellitus, chronic lung disease, chronic renal failure, neuro-psychiatric disorders, heart failure, malignancy and immunosuppression did not differ between the three groups (Table 1).
Infection-related risk factors: On bacteremia day, all patients were on mechanical ventilation and had at least one CVC. Patients that died either early or later revealed more severe organ dysfunction, based on SOFA score calculation compared to survivors (3.7±5 vs 14±2.9 vs 10.7±2.9, p<0.001). The proportion of patients already receiving corticosteroids (8% vs 84.6% vs 31.8%, p<0.001) or being on renal replacement treatment (12% vs 61.5% vs 40.9%, p=0.006) was significantly higher in the fulminantly fatal group compared to other two study groups (Table 1).
Table 1: Risk factors for fulminant sepsis of XDR-KP bacteremic patientsa
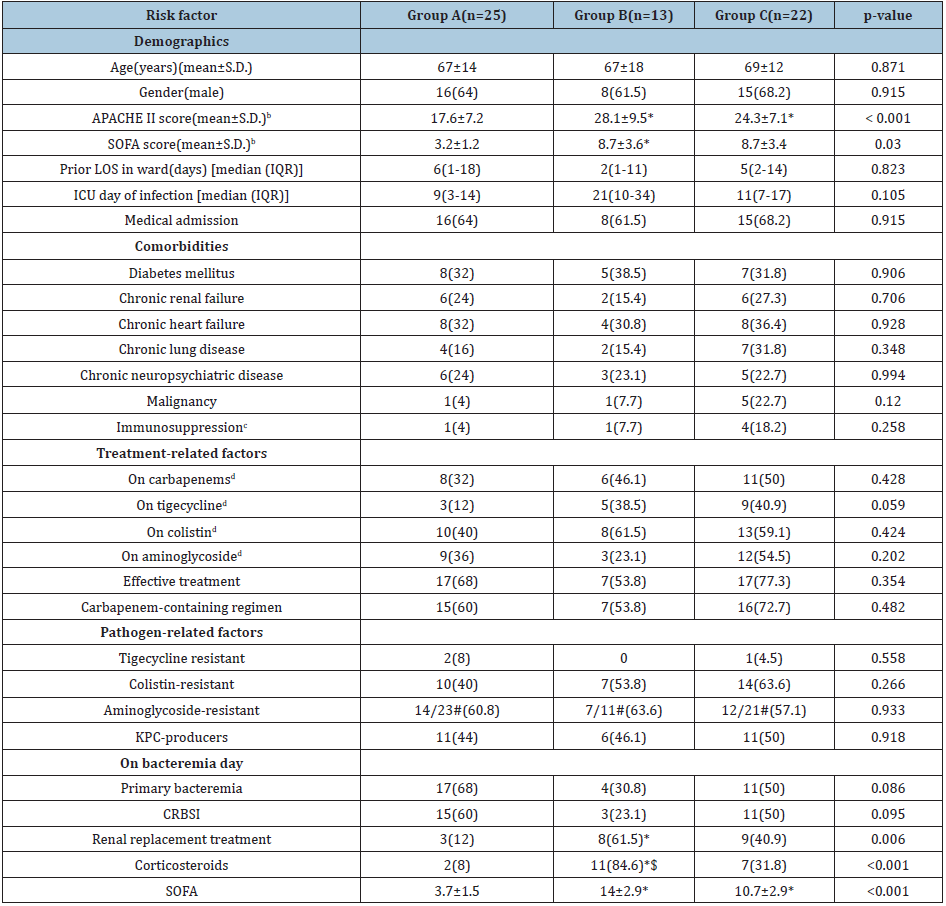
*Significantly different compared to Group A $Significantly different compared to Group C #Among available data XDR-KP, extensively drug-resistant Klebsiella pneumoniae; S.D., standard deviation; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; LOS, length of stay; IQR, interquartile range; ICU, intensive care unit; CRBSI, catheter-related bloodstream infections. aData are no (%) of patients unless otherwise stated bAt ICU admission cNeutropenia (neutrophil count< 1000/mm3), immunosuppressant medication (including corticosteroids), splenectomy dPrior or simultaneously with bacteremia onset
Source of bacteremia: Concerning the source of bacteremia, prevalence of primary bacteremias (including catheter-related) was higher in survivors’ group (Table 1). However, the difference marginally did not reach statistical significance (68% vs 30.8% vs 50%, p=0.086). In regard to the origin of secondary bacteremias, half of the episodes were originated from hospital-acquired pneumonia (HAP) or ventilator-associated pneumonia (VAP) (14/28), with intra-abdominal infections (IAIs) accounting for the second cause (9/28) and skin and skin-structure infections (SSSIs) the third cause (3/28). Notably, all IAIs and SSSIs were encountered in the groups with fatal outcome.
Pathogen-related risk factors: High rates of colistin resistance were documented (51.7%) among XDR -Kp isolates. However, the presence of colistin resistance did not have impact on bacteremia outcome (40 % vs 53.8% vs 63.6%). No pan-resistant strain was identified, whereas only 3 tigecycline- resistant isolates were isolated in the whole study population. Characterization of the carbapenemase content of 60 XDR -Kp bloodstream isolates has revealed the presence of KPC (n=28), OXA-48 (n=18), VIM (n=9), NDM (n=3), OXA-48+VIM (n=1) and OXA-48+NDM (n=1) producers. The rate of KPC-producers was similar among the three outcome groups (Table 1).
Table 2: Characteristics of patients with fulminant XDR-KP bacteremia.
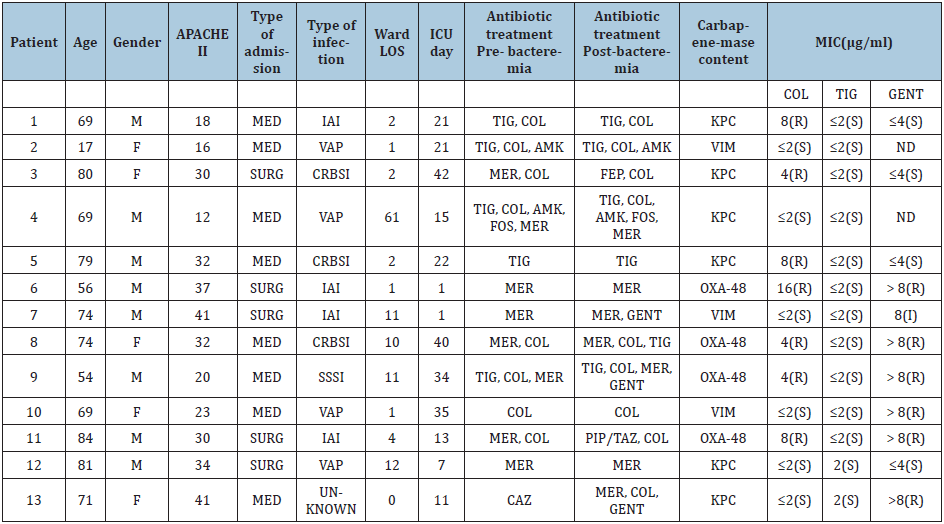
XDR -KP, extensively drug-resistant Klebsiella pneumoniae; M, male sex; F, female; APACHE, Acute Physiology and Chronic Health Evaluation; MED, medical; SURG, surgical; IAI, intra-abdominal infection; VAP, ventilator-associated pneumonia; CRBSI, catheter-related bloodstream infection; SSSI, skin and skin structure infection; LOS, length of stay; ICU, intensive care unit; TIG, tigecycline; COL, colistin; AMK, amikacin; MER, meropenem; FEP, cefepime; CAZ: ceftazidime, FOS, fosfomycin; GENT, gentamicin; PIP/TAZ, piperacillin/tazobactam; MIC, Minimum Inhibitory Concentration; ND: no data
Treatment-related risk factors: Regarding treatment-related factors, there were no differences in prescription of tigecycline, colistin, aminoglycoside or carbapenems prior or on the day of bacteremia among the patients. Receipt of appropriate treatment, along with carbapenem- containing regimens, were also similar between the three outcome groups. Of note, in all cases of catheter-related infections, the CVC was promptly removed. Demographic, microbiological and treatment characteristics of the patients who suffered from fulminant XDR -Kp bacteremia are depicted on Table 2. Multinomial logistic regression analysis has shown that risk factors for fulminant sepsis by XDR-Kp included corticosteroids uptake [odds ratio (OR)=0.002, 95% confidence interval (CI)=0.003-0.196, p=0.001] and immunosuppression [odds ratio (OR)=65.45, 95% confidence interval (CI)=2.4-1785.3, p=0.013] compared with patents with late sepsis (Group C) and corticosteroids uptake [odds ratio (OR) = 0.006, 95% confidence interval (CI)=0.000-0.080, p=0.000] and APACHE II score [odds ratio (OR)=0.776, 95% confidence interval (CI)=0.664-0.907, p=0.001] compared with survivors (Group A).
Comparison of host-, infection-, pathogen- and treatment-related characteristics between XDR -Kp and XDR-Ab fulminant bactreremias
Table 3: Comparison of risk factors for fulminant sepsis between bacteremic patients with XDR-Kp and XDR-Aba

XDR-Kp, extensively drug-resistant Klebsiella pneumoniae; XDR-Ab, extensively drug-resistant Acinetobacter baumannii; S.D., standard deviation; APACHE, Acute Physiology and Chronic Health Evaluation; SOFA, Sequential Organ Failure Assessment; LOS, length of stay; IQR, interquartile range; ICU, intensive care unit; CRBSI, catheter-related bloodstream infections; HAP/ VAP, hospital acquired pneumonia/ ventilator-associated pneumonia; IAI, intraabdominal infection; SSSI, skin and skin-structure infection ; A-W, autumn winter #Among available data aData are no. (%) of patients unless otherwise stated bAt ICU admission cNeutropenia (neutrophil count <1000/mm3), immunosuppressant medication (including corticosteroids), splenectomy dPrior or simultaneously with bacteremia onset
During the study period 13 XDR -Kp and 14 XDR-Ab fulminantly fatal bacteremias were recorded. No differences regarding age, gender, comorbidities, severity scores and cause of ICU admission were identified. As shown by ANOVA analysis (Table 3), fulminant bactreremias due to XDR -Kp emerged significantly later during ICU hospitalization compared to corresponding bacteremias due to XDR-Ab (median ICU day: 21 vs 8, p=0.024). Regarding pathogen-related factors, isolation of colistin resistant strains was 2-fold more frequent in XDR -Kp bacteremias (53.8 vs 21.4%). However, this difference did not reach statistical significance. Tigecycline resistance, to the contrary, concerned significantly more frequently the XDR-Ab strains (0 vs 35.7%, p=0.041). Pandrug resistant strains observed only in the XDR-Ab group. Concerning the source of infection, patients with XDR-Ab isolation showed a higher prevalence of primary bacteremias (including CRBSIs). Pneumonia (HAP or VAP) and IAIs accounted for the majority of secondary bacteremias in both groups. No differences were observed in the two study groups regarding the use of carbapenems, tigecycline or colistin, prior or on the infection day. Administration of effective treatment was also similar between the two bacteremic groups. On bacteremia day, severity of infection and multi-organ dysfunction, as measured by SOFA score, were comparable between the study groups. Nevertheless, implementation of steroid therapy and renal replacement treatment were 2-fold (84.6% vs 42.9%, p=0.046) and 3-fold higher (61.5% vs 21.4%, p=0.084) in patients with XDR-Kp fulminant bacteremias, respectively. Binary logistic regression analysis has shown that risk factors for XDR-Kp v.s. XDR-Ab fulminant sepsis included prior ICU LOS on infection day [odds ratio (OR)=1.112, 95% confidence interval (CI)=1.004-1.231, p=0.042] and corticosteroids uptake [odds ratio (OR)=9.025, 95% confidence interval (CI)=1.020-79.814, p=0.048].
Discussion
Klebsiella pneumoniae is a bacterial pathogen that reveals great adroitness at developing antibiotic resistance. Bloodstream infections due to CR-Kp have been associated with particularly high mortality, with previous studies reporting overall mortality rates of 48- 53% and infection-related mortality rates of 34- 38% (15,16). Infection-related mortality involves several factors including host defense, infection location and source control, virulence of the pathogen and the efficacy of forthcoming antimicrobial agents. Through the present study, we investigated various factors, either host- or pathogen- and infection-related, in order to identify potential risk factors that could predetermine a fulminant course of infection. Crude and infection mortality reached 58.3 % and 35% respectively. In particular, 13/35 deaths came about within 48h after bacteremia onset, indicating a fulminant course of sepsis. An exaggerated hyperinflammatory response has been implicated as the cause of early sepsis mortality. Elements of sepsis expression involve host factors, bacterial burden and virulence factors of individual strains [15-17]. Regarding host factors, the individual response to septicemia is variable depending on the patient’s immune response, age, nutritional status and coexisting conditions [17]. Previous studies have identified that chronic comorbidities were more frequent among patients with CR-Kp bacteremias but not independent risk factors for CR-Kp bloodstream infections [18]. Similarly, chronic comorbidities did not prove to affect mortality rate of bacteremic Kp ICU patients [19]. However, it has been shown that factors such as underlying disease and comorbidities play a more important role on mortality than appropriate empiric treatment with multidrug-resistant Gram-negative bacteria [20]. In our study, no chronic comorbidity was identified as risk factor for unfavorable outcome, either early or late. Nevertheless, patients with grim outcome presented at ICU admission with higher illness severity based on APACHE II score. Additionally, organ dysfunction was also more severe in the two study groups with poor outcome. Klebsiella pneumoniae often colonizes the respiratory or intestinal tract and can cause invasive infection when immunity is compromised. A recent immune function study demonstrated that a correlation between significantly impaired immune function and disease severity scores [21]. Our findings are in accordance with previous publications [22,23] and suggestive of severely ill patients’ incompetence to counteract Klebsiella pneumoniae infections. Moreover, patients that died early or late manifested more severe septic profile, based on SOFA score on bacteremia day, compared to survivors. Patients suffered from fatal course of bacteremia, revealed significantly higher rates of renal replacement therapy and corticosteroids prescription on infection day. Notably, all the aforementioned patients presented with septic shock, which is in accordance with the study of Tumbarello et al [23]. Treatment with corticosteroids and dialysis are among risk factors for acquisition of Kp nosocomial bacteremia, along with diabetes, chronic liver disease and transplantation [1,22]. Furthermore, a highly significant suppression of pro-inflammatory cytokine release has been observed in patients receiving stress doses of hydrocortisone, indicating further weakening of immune response [21]. Acute renal failure is a common complication of severe sepsis and is considered not only as an indicator of disease severity but also as independent risk factor for death [24].
The source of bacteremia may affect its clinical course and outcome. Effective and timely infection source control is a cornerstone in the treatment of infectious diseases [25]. Prompt removal of an infected CVC is an effective source control of bacteremia and may have affected bacteremias’ clinical course in the survivor’s group, where the prevalence of catheter-related bloodstream infection was the highest. Similarly, Patel et al concluded that removal of the focus of infection was independedly associated with patient survival [15]. The role of invasive devices has been implicated in colonization and subsequent infection, through destroying the integrity of skin and mucosa [26]. Since our whole study population had at least one CVC, arterial catheter, urinary catheter and was on mechanical ventilation through endotracheal tube or tracheostoma, we did not test their effect on bacteremias outcome. The appropriate and prompt use of antimicrobials is another significant element of the effective treatment of infections. Timely effective antimicrobial treatment has been associated with survival [16,23]. In our cohort, the percentage of appropriate treatment within 48h ranged between 53.8%-77.3% and did not significantly differ between the outcome groups. Additionally, we tested for the interaction between outcome and previously administered carbapenems, tigecycline, colistin and aminoglycoside. Administration of colistin and carbapenems prior or simultaneously with bacteremia was similar between the outcome groups. Nevertheless, rate of tigecycline prescription was higher in Group C with late deaths compared to survivors, with marginally insignificant difference. Α recent study demonstrated that previous carbapenem exposure was independent risk factor for CR-Kp bacteremia acquisition, whereas, patients treated with tigecycline after bacteremia onset demonstrated significantly higher mortality compared with patients received other antibiotics (51.2% vs 12.2%, p< 0.001) (22). Previous meta-analyses documented diverse results regarding mortality in patients who received tigecycline [27,28]. Recently, Machuca et al. [29] demonstrated that combination therapy (mostly tigecycline-containing regimens) was associated with reduced mortality in patients with bacteremia due to colistin-resistant CR-Kp in patients with septic shock [29]. Given the fact that almost all XDR-Kp strains in this patient series were tigecycline sensitive, the poor outcome may be attributed to tigecycline’s bacteriostatic action and low serum concentration rather than inappropriate treatment [30]. Previous workers concluded that carbapenem-containing combination schemes were significantly associated with lower mortality rates than carbapenem-sparing regimens, in cases where the meropenem MIC ≤ 8μg/ml [23]. However, this positive carbapenem effect could not apply to our cohort, since all XDR-Kp had carbapenem MICs >8μg/ml. In the current study, we evaluated the antibiotic resistance determinants of XDR-Kp bacteremias and their potential relation with clinical outcome, especially with a fulminant course of bacteremia. Notably, only three strains in the whole population were tigecycline-resistant and no pandrug resistance was observed. Even though patients with poor outcome showed almost 1.5-fold higher rates of colistin resistance compared with survivors, this difference did not reach statistical significance, probably due to the small size sample. The association between colistin resistance and mortality has been demonstrated and may be related to the decreased colistin susceptibility of the organism per se and the resulted limited therapeutic options [31]. Molecular characterization of the XDR-Kp isolates revealed predominance of bla KPC producers, Previous publications have displayed KPC- producers Klebsiella pneumoniae to be an independent risk factors of mortality [32]. Carriage of other drug-resistant genes that lead to marked drug resistance along with pathogens’ ability to persist and spread, may contribute to more pronounced invasiveness and subsequent mortality [33]. Nevertheless, in our cohort no differences in KPC to non-KPC ratio were identified among the three outcome groups. In the present study, we compared the clinical features and infection related characteristics of fulminantly fatal bacteremias due to XDR-Kp or XDR-Ab strains. The patients of both groups showed similar demographics and co-morbidities. Nevertheless, XDR-Kp patients developed the corresponding fatal bacteremias significantly later during ICU hospitalization than XDR-Aba group. In the ICU environment there are several potential sources of acquisition of these pathogens. Gastrointestinal colonization is likely a common and significant reservoir in terms of transmission and infection [34]. In a recent study that compared septic shock due to multidrug-resistant Acinetobacter baumannii or carbapenemase-producing Klebsiella pneumoniae, hospitalization in the previous 90 days was among the distinguishing features of KPC-Kp infections [35]. Nevertheless, Acinetobacter’s baumannii ability to survive for long periods of time on inanimate surfaces in the patients’ vicinity might be involved in the early development of the corresponding fulminant bacteremias, through transmission via healthcare workers’ hands [36]. We found an association between steroid administration and XDR-Kp fulminant bacteremia. On the other hand, previous researchers have reported an association between steroid therapy and development of multidrug- resistant Ab infection but the interpretation of this association was not established [35]. In our series, the use of steroids in the cases of complicated IAIs, SSSI and VAPs, in order to reduce inflammation might be encountered in the development of XDR-Kp fulminant bacteremias. Glucocorticoids have multiple anti-inflammatory and immunosuppressive effects. They suppress macrophage production of Interleukin-1, Interleukin-6, tumor necrosis factor and leukotrienes and virtually affect all immune cells [37]. KPC-producing isolates seem to be highly virulent in a low tumor necrosis factor alpha release environment [38]. Therefore, an immunoparalysis induction mechanism may be the link between increased use of steroids and predominance of highly virulent XDR-Kp strains. Extensively-drug resistant Ab isolates in patients with fulminant bacteremias were significantly tigecycline-resistant compared to the corresponding XDR-Kp isolates. The latter, on the contrary, revealed 2.5-fold higher colistin-resistance, although the difference did not reach statistical significance. These aforementioned differences in XDR-Aba and XDR-Kp strains may reflect the different resistance patterns encountered in our institution. The limited therapeutic options against infections caused by XDR bacteria have led to increased use of colistin and tigecycline and thus promoting emergence of resistance. Colistin resistance augmentation may further contribute to treatment failure of infections caused by XDR-Kp [39]. Antimicrobial resistance and virulence represent significant factors in the pathogenicity of K. pneumoniae. Although there is largely non-overlapping between antimicrobial resistance and hypermucoviscous hypervirulent phenotype of Kp, a few recent studies reported some cases of KPC-producing hypermucoviscous strains [40]. In our series, almost half of colistin resistant strains encountered in fulminant bacteremias were KPC-producers. The present study has some limitations that should be considered. This is a single-center study and the results might not be generalized, since local susceptibility patterns might differ among other institutions. The observational and retrospective nature of the study brings about an intrinsic limitation. The relative low number of patients, especially those with fulminant course of bacteremias, may have underestimated the causal role of certain risk factors. Nevertheless, this study is a real-life clinical experience that provides useful data regarding characteristics and treatment challenges of bacteremias due to XDR Gram negative pathogens in critically ill patients. In conclusion, through the present study we attempted to identify differential risks that could predict mortality of XDR-Kp infected patients. We demonstrated that higher disease severity and administration of corticosteroids and renal replacement therapy posed patients at higher risk for fulminantly fatal course of XDR-Kp bacteremia. Receipt of appropriate treatment did not affect outcome. The comparison of fulminant cases due to extensively-drug resistant Klebsiella pneumoniae or Acinetobacter baumannii strains revealed that XDR-Kp bacteremias emerged significantly later during ICU hospitalization. Rates of corticosteroids and renal replacement therapy administration were also higher when compared to XDR-Ab patients. Pandrug resistant strains observed only in the XDR-Ab group. The aforementioned findings signify the difficulties with handing infections caused by extensively-drug resistant Kp or Ab, considering the complexity of critically ill patients and the extremely limited therapeutic options. Further studies in larger samples of patients that would analyze specific virulence determinants of XDR strains along with investigation of potential de-arrangement of host immune system are needed, in order to improve treatment strategies. However, continuous surveillance in combination with strict infection control measures is remaining the cornerstone for restraining infection rates caused by XDR bacteria and thus reduce mortality.
References
- Paczosa MK, Mecsas J (2016) Klebsiella pneumoniae: going on the offense with a strong defense. Microbiol Mol Biol Rev 80(3): 629-661.
- Tian L, Tan R, Chen Y, Sun J, Liu J, et al. (2016) Epidemiology of Klebsiella pneumoniae bloodstream infections in a teaching hospital: factors related to the carbapenem resistance and patient mortality. Antimicrob Resist Infect Control 5:48.
- Trecarichi EM, Pagano L, Martino B, Candoni A, Di Blasi R, et al. (2016) Haematologic malignancies associated bloodstream infections surveillance (HEMABIS) registry-Sorveglianza Epidemiologica Infezioni Funginein Emopatie Maligne (SEIFEM) group Italy. Bloodstream infections caused by Klebsiella pneumoniae in onco-hematological patients: Clinical impact of carbapenem resistance in a multicentre prospective survey. Am J Hematol 91(11): 1076-1081.
- Xu L, Sun X, Ma X (2017) Systematic review and meta-analysis of mortality of patients infected with carbapenem-resistant Klebsiella pneumoniae. Ann Clin Microbiol Antimicrob 16(1): 18.
- Katsiari M, Mavroidi A, Platsouka ED, Nikolaou C (2018) Extensively drug-resistant acinetobacter baumannii bacteremia in a multidisciplinary intensive care unit during a 6-year period: risk factors for fulminant sepsis. J Glob Antimicrob Resist 14: 51-57.
- Magiorakos AP, Srinivasan A, Carey RB, Carmeli Y, Falagas ME, et al. (2012) Multidrug-resistant, extensively drug-resistant and pandrug-resistant bacteria: an international expert proposal for interim standard definitions for acquired resistance. Clin Microbiol Infect 18(3): 268-281.
- Rhodes A, Evans LE, Alhazzani W, Levy MM, Antonelli M, et al. (2017) Surviving sepsis campaign: international guidelines for management of sepsis and septic shock: 2016. Intens Care Med 43(3): 304-377.
- Daviaud F, Grimaldi D, Dechartres A, Charpentier J, Geri G, et al. (2015) Timings and causes of death in septic shock. Ann Intens Care 5(1): 16.
- Clinical and Laboratory Standards Institute (CLSI) (2018) Performance standards for antimicrobial susceptibility testing, USA.
- Tsakris, A, Poulou A, Pournaras S, Voulgari E, Vrioni G, et al. (2010) A simple phenotypic method for the differentiation of metallo-lactamases and class A KPC carbapenemases in Enterobacteriaceae clinical isolates. J Antimicrob Chemother 65(8): 1664-1671.
- Glupczynski Y, Evrard S, Ote I, Mertens P, Huang TD, et al. (2016) Evaluation of two new commercial immunochromatographic assays for the rapid detection of OXA-48 and KPC carbapenemases from cultured bacteria. J Antimicrob Chemother 71(5): 1217-1222.
- Cointe A, Bonacorsi S, Truong J, Hobson C, Doit C, et al. (2018) Detection of carbapenemases-producing Enterobacteriaceae in positive blood culture using immunochromatographic RESIST-4 O.K.N.V assay. Antimicrob Agents Chemother 62(12): e01828-18.
- Avgoulea K, Di Pilato V, Zarkotou O, Sennati S, Politi L, et al. (2018) Characterization of extensively drug-Resistant or pandrug-resistant sequence type 147 and 101 OXA-48-Producing Klebsiella pneumoniae causing bloodstream infections in patients in an intensive care unit. Antimicrob Agents Chemother 62(7): e02457-17.
- Mavroidi A, Katsiari M, Likousi S, Palla E, Roussou Z, et al. (2016) Characterization of ST258 colistin-resistant, blaKPC-producing Klebsiella pneumoniae in a Greek Hospital. Microb Drug Resist 22(5): 392-398.
- Patel G, Huprikar S, Factor SH, Jenkins SG, Calfee DP (2008) Outcomes of carbapenem-resistant Klebsiella pneumoniae infection and the impact of antimicrobial and adjunctive therapies. Infect Control Hosp Epidemiol 29(12): 1009-1106.
- Zarkotou O, Pournaras S, Tselioti P, Dragoumanos V, Pitiriga V, et al. (2011) Predictors of mortality in patients with bloodstream infections caused by KPC-producing Klebsiella pneumoniae and impact of appropriate antimicrobial treatment. Clin Microbiol Infect 17(12): 1798-1803.
- Hotchkiss RS, Karl IE (2003) The pathophysiology and treatment of sepsis. N Engl J Med 348(2): 138-150.
- Hussein K, Raz pasteur A, Finkelstein R, Neuberger A, Shachor MY, et al. (2013) Impact of carbapenem resistance on the outcome of patients’ hospital-acquired bacteraemia caused by Klebsiella pneumoniae. J Hosp Infect 83(4): 307-313.
- Zheng X, Wang JF, Xu WL, Xu J, Hu J (2017) Clinical and molecular characteristics, risk factors and outcomes of carbapenem-resistant Klebsiella pneumoniae bloodstream infections in the intensive care unit. Antimicrob Resist Infect Control 6: 102.
- Vardakas KZ, Rafailidis PI, Konstantelias AA, Falagas ME (2013) Predictors of mortality in patients with infections due to multi-drug resistant Gram-negative bacteria: the study, the patient, the bug or the drug? J Infect 66(5): 401-414.
- Feuerecker M, Sudhoff L, Crucian B, Pagel JI, Sams C, et al. (2018) Early immune anergy towards recall antigens and mitogens in patients at onset of septic shock. Scientific Reports 8: 1754.
- Xiao T, Yu W, Niu T, Huang C, Xiao Y (2018) A retrospective, comparative analysis of risk factors and outcomes in carbapenem-susceptible and carbapenem-non susceptible Klebsiella pneumoniae bloodstream infections: tigecycline significantly increases the mortality. Infect Drug Res 11: 595-606.
- Tumbarello M, Viale P, Viscoli C, Trecarichi EM, Tumietto F, et al. (2012) Predictors of mortality in bloodstream infections caused by Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae: importance of combination therapy. Clin Infect Dis 55(7): 943-950.
- Oppert M, Engel C, Brunkhorst FM, Bogatsch H, Reinhart K, et al. (2008) Acute renal failure in patients with severe sepsis and septic shock- a significant independent risk factor for mortality: results from the German prevalence study. Nephrol Dial Transplant 23(3): 904-909.
- Marshall JC (2010) Principles of source control in the early management of sepsis. Curr Infect Dis Rep 12(5): 345-353.
- Schechner V, Kotlovsky T, Kazma M, Mishali H, Schwartz D, et al. (2013) Asymptomatic rectal carriage of bla KPC producing carbapenem-resistant Enterobacteriaceae: who is prone to become clinically infected? Clin Microbiol Infect 19(5): 451-456.
- Shen F, Han Q, Xie D, Fang M, Zeng H, et al. (2015) Efficacy and safety of tigecycline for the treatment of severe infections diseases: an updated meta-analysis of RCT’s. Int J Infect Dis 39: 25-33.
- Ni W, Han Y, Liu J, Wei C, Zhao J, et al. (2016) Tigecycline treatment for carbapenem-resistant Enterobacteriaceae infections: A systematic review and meta-analysis. Medicine (Baltimore) 95(11): e3126.
- Machuca I, Gutiérrez B, Garcia AI, Rivera EF, Cano A, et al. (2017) Mortality associated with bacteremia due to colistin-resistant Klebsiella pneumoniae with high-level meropenem resistance importance of combination therapy without colistin and carbapenems. Antimicrob Agents Chemother 61(8): e00406-17.
- Govern PC, Wible M, Tahtawy A, Biswas P, Meyer RD (2013) All-cause mortality imbalance in the tigecycline phase 3 and 4 clinical trials. Int J Antimicrob Agents 41(5): 463-467.
- Rojas LJ, Salim M, Cober E, Richter SS, Perez F, et al. (2017) Colistin resistance in carbapenem-resistant Klebsiella pneumoniae: Laboratory detection and impact on mortality. Clin Infect Dis 64(6): 711-718.
- Mouloudi E, Protonotariou E, Zagorianou A, Iosifidis E, Karapanagiotou A, et al. (2010) Bloodstream infections caused by metallo-β-lactamase/Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae among intensive care unit patients in Greece: risk factors for infection and impact of type of resistance on outcomes. Infect Control Hosp Epidemiol 31(12): 1250-1256.
- Villegas MV, Lolans K, Correa A, Kattan JN, Lopez JA, et al. (2007) First identification of pseudomonas aeruginosa isolates producing a KPC-type carbapenem-hydrolyzing β-lactamase. Antimicrob Agents Chemother 51(4): 1553-1555.
- Dorman MJ, Short FL (2017) Genome watch: Klebsiella pneumoniae: when a colonizer turns bad. Nat Rev Microbiol 15(7): 384.
- Russo A, Guliano S, Ceccarelli G, Alessandri F, Giordano A, et al. (2018) Comparison of septic shock due to multidrug-resistant Acinetobacter baumannii or Klebsiella pneumoniae carbapenemase-producing Klebsiella pneumoniae in intensive care unit patients. Antimicrob Agents Chemother 62(6): e02562-17.
- Getchell WSI, Donowitz LG, Gröschel DH (1989) The inanimate environment of an intensive care unit as a potential source of nosocomial bacteria: Evidence for long survival of Acinetobacter calcoaceticus. Infect Control Hosp Epidemiol 10(9): 402-407.
- Youssef J, Novosad SA, Winthrop KL (2016) Infection risk and safety of corticosteroid use. Rheum Dis Clin North Am 42(1): 157-176.
- Pantelidou IM, Galani I, Georgitsi M, Daikos GL, Giamarellos BEJ (2015) Interaction of Klebsiella pneumoniae with the innate immune system vary in relation to clone and resistance phenotype. Antimicrob Agents Chemother 59(11): 7036-7043.
- Capone A, Giannella Μ, Fortini D, Giordano A, Meledandri M, et al. (2013) High rate of colistin resistance among patients with carbapenem-resistant Klebsiella pneumoniae infection accounts for an excess of mortalit. Clin Microbiol Infection 19(1): 23-30.
- Zhang R, Lin D, Chan EW, Gu D, Chen GX, et al. (2016) Emergence of carbapenem-resistant serotype K1 hypervirulent Klebsiella pneumoniae strains in China. Antimicrob Agents Chemother 60(1): 709-711.
© 2020 Maria Katsiari. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






