- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Bioengineered Immunotherapy Techniques for the Treatment of Select Secondary Immunodeficiency Disorders
Alyssa T Dalloo* and Mirjana Pavlovic
Florida Atlantic University
*Corresponding author: Alyssa T Dalloo, Florida Atlantic University, USA
Submission: December 07, 2019; Published: December 12, 2019

ISSN 2578-0190 Volume3 issues3
Abstract
The inner workings of the immune system are quite complex. Each component works diligently in order to protect us from invaders such as pathogens: viruses, microbes, and fungi. Unfortunately, the immune system may become dysfunctional, which can result in secondary immunodeficiency diseases. The treatments available for these disorders and diseases of the immune system do not typically serve to cure, and much too often the side effects associated can exacerbate the conditions. As an alternative, the area of immunotherapy seeks to remedy the limitations of today’s medicine. Through this review, insight into the current conventional treatments available for select secondary immunodeficiency diseases will be provided, particularly in Human Immunodeficiency Virus disease and Systemic Lupus Erythematosus. The modern immunotherapy approaches that are underway will then be analyzed in their capacity and potential to treat these defects of the immune system.
Keywords: Immune system; Secondary immunodeficiency disorder; Autoimmune disease; Acquired immune deficiency; Immunotherapy; Human immunodeficiency virus; Systemic lupus erythematosus
Introduction
The very framework of our immune system consists of organs, tissues, and cells that interact systematically to block the passage of foreign bodies and prevent disease. This defense network also can recognize and destroy said molecules, in addition to abnormal cells that originate from an individual’s own tissues. In other words, the immune system is designed to differentiate between self (healthy and unhealthy cells) and non-self (invader microbes). Inclusively, anything that initiates such a response is deemed an antigen. In some individuals, however, the immune system may become confused and direct their destruction on healthy tissues or cells that would be considered harmless otherwise. This leads to disorders and diseases of the immune system, such as autoimmune diseases and allergies [1-6]. Depending on the type of disorder and how it was acquired, there are a multitude of treatments available. Yet, most therapies are intended to provide relief from symptoms and prolong the infancy stage of the disease, rather than provide a cure. There has been a recent influx of research in the realm of Immunotherapy. Immunotherapy seeks to enhance or suppress an individual’s immune response to weaken or eradicate the disease of concern. This method tends to be entwined with the treatment of cancers [1]. However, the techniques used can also be applied to some disorders and diseases of the immune system. To gain an appreciation of the deficits which may occur, it is pivotal to first understand how a healthy immune system should operate. Once this baseline has been established, a comparison can be made with individuals that suffer from select secondary immunodeficiencies, including subsets from the acquired immune deficiency and autoimmune disease categories. An examination on the modern research in immunotherapy techniques which are being incorporated in the treatment of these secondary immunodeficiencies will then be conducted. Consequently, with respect to the disorders reviewed, the possible enhancements and developments that can be applied to further improve the effectiveness of the treatments will be analyzed.
Patient N
Patient N will serve as a representative of the population that has a healthy and fully operational immune system. Since numerous deficits of the immune system occur at the cellular and molecular level, the basis of review will be focused on those components.
Innate Immunity
In Patient N, the immune responses and mechanisms of the immune system can be characterized as either innate immunity or adaptive immunity. Through innate immunity, a nonspecific defense mechanism is invoked when foreign particles are initially encountered. The immune system’s first line of defense includes the skin, cornea, and mucosa of the respiratory and digestive tracts. When this physical barrier has been breached, Toll-Like Receptors (TLRs) are able to collectively recognize general dangeror pathogen-associated-patterns of microbes that may enter. This recognition in turn triggers expression of genes and thus the innate responses by the immune cells. The innate cell types involved, and their respective specialized functions are summarized in Figure 1; [2-6].
Figure 1: Innate immune cells and their functions- Patient N.
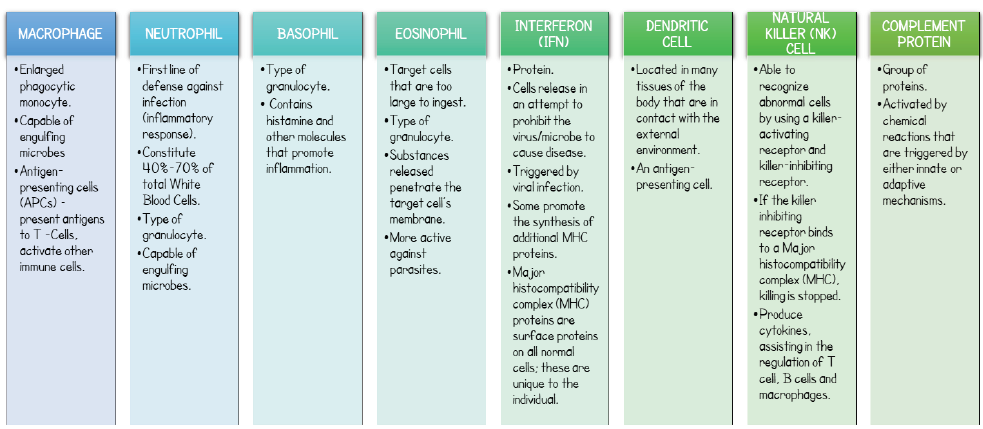
Adaptive Immunity
In addition to the defense mechanisms supplied by the innate system, there is further protection provided through the adaptive system for Patient N. In adaptive immunity, the cells involved are more specialized. Instead of recognizing general patterns, these cells can recognize specific signals. The antigens that were processed by the innate system are presented to the adaptive cells so that appropriate action can be taken. The main types of lymphocytes involved are B cells and T cells. The genes for the B-cell receptors (BCRs) and T-cell receptors (TCRs) undergo recombination during certain cell maturation states. This randomization results in a diversity of receptors. The functions of the cells involved in the adaptive system are summarized in Figure 1. In addition to the functions specified in Figure 1, B cells and T cells both undergo processes of positive and negative selection. As a result of positive selection, the cell can mature and thus survives, whereas in negative selection, the cell undergoes programmed cell death. The latter typically occurs if the immature cells bind to selfantigens [2-6]; Figure 2.
Figure 2: Adaptive immune cells and their functions- Patient N.

Through Patient N, a baseline of the appropriate functioning of the immune system has been developed. With this information, insight into what occurs in select secondary immunodeficiencies can be better appreciated.
Acquired immune deficiency diseasesAcquired Immune Deficiency Diseases are attained and developed as a result of certain external interactions. Under this subset of secondary immunodeficiency disorders are conditions including: Acquired Immunodeficiency Syndrome (AIDS) and Human Immunodeficiency Virus (HIV), in addition to Graft Versus Host Disease. The first will be reviewed in detail [1].
Patient H
Patient H was born with a similar immune system functioning to the same degree as that of Patient N. However, Patient H has contracted HIV. The Human Immunodeficiency Virus (HIV) is transmitted through the exchange of select bodily fluids including breast milk, blood, semen, vaginal, cervical and rectal secretions, with an HIV-positive individual. HIV ravages the immune system by depleting the number of CD4+ T cells, which can debilitate the system and allow for further infections and cancers to prosper. With the development of these opportunistic infections, the individual has reached Stage 3 of the virus: Acquired Immunodeficiency Syndrome (AIDS). There are currently no cures available, however the current treatment involves the use of antiretroviral therapy (ART). When taken as prescribed, ART has the capability to slow down the progression of the virus from proliferating into the deadly last stage [7,16]. Though ART has been a useful treatment of HIV, there have often been cases of remission, and even death. This is due to the virus’ proviral DNA remaining latent in CD4+ T cells. As a result, a life-long administration of ART would be necessary to prohibit the reactivation of the virus and hinder the development of AIDS. However, with the continued use of ART, the virus continues to proliferate which can open the doorway to additional non-AIDS related cancers and comorbidities; a possible fate for Patient H [8]. As a possible alternative, the current research and studies available in the field of immunotherapy provide a pleather of potential treatments Table 1.
Table 1: Modern immunotherapy techniques reviewed- Patient H.
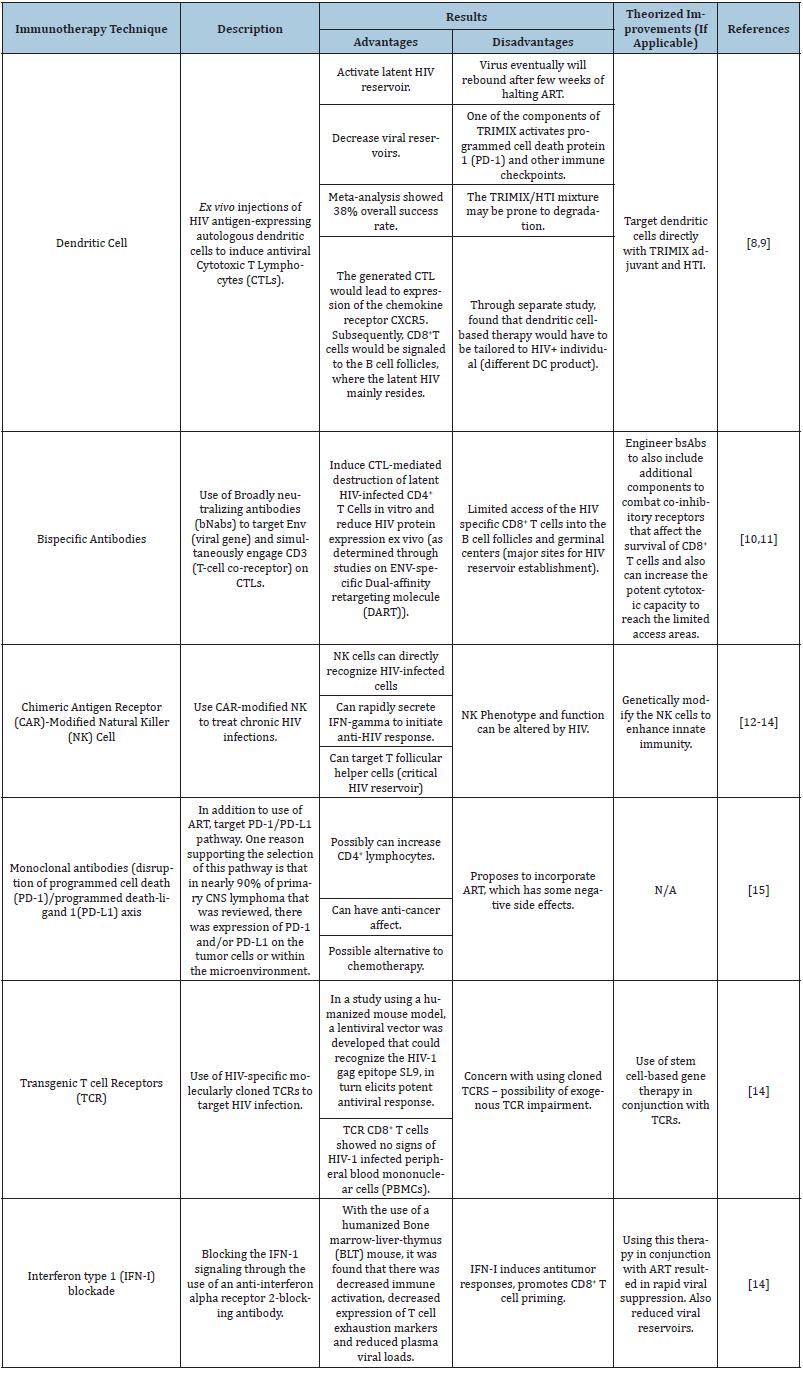
Referring to Table 1, it is apparent that there is the need to determine how to eradicate the HIV reservoirs. In addition, there seems to be a debate on whether it is still necessary to use ART for the reviewed immunotherapies. Each technique is promising, but further research will be necessary in order to confirm the validity and applicability.
Autoimmune diseasesUnlike acquired immune deficiency diseases, the culprit behind the dysfunction in autoimmune diseases is not always known. For diseases and disorders of this nature, the immune system can start to attack healthy tissues, rendering certain functions altered and at times so damaged that it can no longer function at all. For the purposes of this review, a deeper look into Systemic Lupus Erythematosus will be analyzed, as represented through Patient L [1, 9-16].
Patient L
Patient L has been diagnosed with Systematic Lupus Erythematosus (SLE), an inflammatory disease. This chronic disease can be mild or severe, in which it causes inflammation in connective tissues. SLE can affect many organs and systems. At its most severe, SLE can lead to inflammation of the kidneys, nervous system, brain and/or arteries, which can be life threatening. It is theorized that the disease is onset by a combination of genetic mutations and environmental factors, however, the exact cause has not been fully uncovered. Currently there is no cure for SLE. However, the disease is typically treated with a combination of immunosuppressants and lifestyle changes. The exact treatment depends on the symptoms experienced by the patient and the organs that are affected. The available therapies seek to control the production of autoantibodies, in addition to the levels of INF-1 and various chemo-attractants [17,18]. As a promising alternative, the current research and studies available in the field of immunotherapy propose some potential treatments [19-24].
Referring to Table 2, it can be observed that there are contrasting viewpoints on what is the appropriate molecule/cell to target in order to reduce the responses experienced by individuals with SLE, such as Patient L. Each are still in the preliminary phases and will require additional experimentation.
Table 2: Modern immunotherapy techniques reviewed- Patient L.
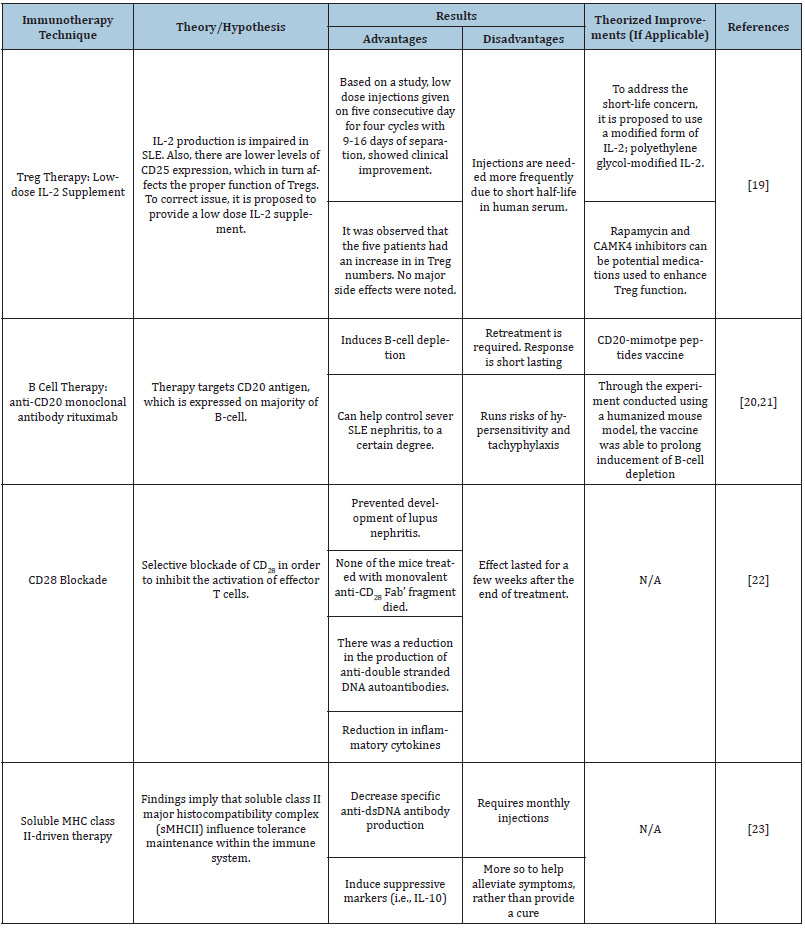
Conclusion
Through the analysis of Patients N, H and L, an intimate look into what occurs in the immune system in a healthy individual versus when the person has a secondary immunodeficiency disease was observed. The slightest disruption to the framework of the immune system can result in damage to healthy tissues leading to cancers, infectious diseases, and autoimmune diseases. The immunotherapy available for HIV seeks to eventually eradicate the virus by targeting the immune cells that are harvesting the deadly latent strands. In addition, many are aiming to reduce or even cease the use of ART as a life-long treatment. For SLE, the immunotherapies being researched are still trying to determine which immune cell is the most appropriate to suppress. Each category of immunotherapy is promising, however additional research is necessary to confirm many of the advantages that have been described in the articles. Though it may seem that there is still a long way to go, it is imperative that research is continued in these fields. Immunotherapy provides hopes of improving the lives of those affected by these and other debilitating secondary immunodeficiency diseases.
References
- Shannon JB (2005) Immune System Disorders Sourcebook. Detroit MI: Omnigraphics.
- Patton KT, Thibodeau GA (2016) Anatomy & Physiology. Elsevier/Mosby.
- NIAID (2014) Features of an Immune Response.
- NIAID (2014) Immune Cells.
- Delves PJ (2018) Cellular components of the immune system. Allergic Disorders.
- Delves PJ (2018) Molecular components of the immune system. Allergic Disorders.
- CDC (2018) HIV Basics.
- Ali A, Charles RR (2017) A novel anti-HIV immunotherapy to cure HIV. AIDS (3): 447.
- Pontillo A, Reis EC, Silva LT, Duarte AJS, Crovella S, et al. (2016) Dendritic cells used in anti-HIV immunotherapy showed different modulation in anti-HIV genes expression: New concept for the improvement of patients’ selection criteria. Journal of Cellular Immunotherapy 2(2): 85-94.
- Fabozzi G, Pegu A, Koup RA, Petrovas C (2019) Bispecific antibodies: Potential immunotherapies for HIV treatment. Methods 154: 118-124.
- Nishimura Y, Martin MA (2017) Of Mice, macaques, and men: broadly neutralizing antibody immunotherapy for HIV-1. Cell Host & Microbe 22(2): 207-216.
- Liu D, Tian S, Zhang K, Xiong W, Lubaki NM, et al. (2017) Chimeric antigen receptor (CAR)-modified natural killer cell-based immunotherapy and immunological synapse formation in cancer and HIV. Protein & Cell 8(12): 861-877.
- Zenere G, Olwenyi OA, Byrareddy SN, Braun SE (2019) Optimizing intracellular signaling domains for CAR NK cells in HIV immunotherapy: a comprehensive review. Drug Discovery Today 24(4): 983-991.
- Carrillo MA, Zhen A, Kitchen SG (2018) The use of the humanized mouse model in gene therapy and immunotherapy for HIV and cancer. Front Immunol 9: 746.
- Kasi PM, Block MS, Ansell SM (2016) Treatment of HIV/AIDS associated cancers with immunotherapy targeting PD-1/PD-L1 instead of chemotherapy. Medical Hypotheses 86: 129-131.
- Okoye AA, Picker LJ (2013) CD4+ T-cell depletion in HIV infection: mechanisms of immunological failure. Immunological Rev 254(1): 54-64.
- NIH (2019) Systemic lupus erythematosus-Genetics Home Reference NIH.
- CDC (2018) Systemic Lupus Erythematosus (SLE)|CDC.
- Pavlovic MA, Kats M, Cavallo YS (2010) Clinical and molecular evidence for association of SLE with parvovirus B19. Lupus 19(7): 783-792.
- Mizui M, Tsokos GC (2018) Targeting regulatory T cells to treat patients with systemic lupus erythematosus. Front Immunol 17(9): 786.
- Favoino E, Prete M, Marzullo A, Millo E, Shoenfeld Y, et al. (2017) CD20-Mimotope peptide active immunotherapy in systemic lupus erythematosus and a reappraisal of vaccination strategies in rheumatic diseases. Clin Rev Allergy Immunol 52(2): 217-233.
- Lin W, Seshasayee D, Lee WP, Caplazi P, McVay S, et al. (2015) Dual B Cell immunotherapy is superior to individual anti-cd20 depletion or baff blockade in murine models of spontaneous or accelerated lupus. Arthritis & Rheumatology 67(1): 215-224.
- Laurent L, Le Fur A, Bloas RL, Neel M, Mary C, et al. (2017) Prevention of lupus nephritis development in NZB/NZW mice by selective blockade of CD28. Eur J Immunol 47(8): 1368-1376.
- Bakela K, Dimakopoulou M, Batsou P, Manidakis N, Athanassakis I (2018) Soluble MHC class II-driven therapy for a systemic lupus erythematosus murine experimental in vitro and in vivo model. Scand J Immunol 87(3).
© 2019 Alyssa T Dalloo. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






