- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Prevalence and Risk Factors of Chlamydia trachomatis Infection in Female Sex Workers Living in Isfahan, Iran: A Cross-Sectional Study
Ghasem Yadegarfar1, Roya Taleban2, Mohammad Moafi3, Nazila Kassaian4, Behrooz Ataie5, Marjan Meshkati6, Zohre Tahvilian7, Shervin Ghaffari Hoseini8 and Somayeh Haghighipour9*
1Associate Professor in Epidemiology & Biostatistics, Cancer Prevention Research Centre & Epidemiology and Biostatistics Dept. School of Public Health, Isfahan University of Medical Sciences, Isfahan, Iran
2Community Medicine Specialist, Infectious Diseases and Tropical Medicine Research center, Isfahan University of Medical Sciences, Isfahan, Iran
3Ph.D., Acquired Immunodeficiency Research Center, Isfahan University of Medical Sciences, Isfahan, Iran
4PhD. Infectious Diseases and Tropical Medicine Research center, Isfahan University of Medical Sciences, Isfahan, Iran
5Professor of Infectious Diseases, Infectious Diseases and Tropical Medicine Research center, Isfahan University of Medical Sciences, Isfahan, Iran
6General Practitioner, Diseases Control Unit, Isfahan Province Health Centre, Isfahan University of Medical Sciences, Isfahan, Iran
7Researcher, Infectious Diseases and Tropical Medicine Research Centre, Isfahan University of Medical Sciences, Isfahan, Iran
8Ph.D., Infectious Diseases and Tropical Medicine Research Centre, Isfahan University of Medical Sciences, Isfahan, Iran
9Assistant Professor of Infectious Diseases, Infectious Diseases and Tropical Medicine Research Centre, Isfahan University of Medical Sciences, Isfahan, Iran
*Corresponding author: Somayeh Haghighipour, Assistant Professor of Infectious Diseases, Infectious Diseases and Tropical Medicine Research Centre, Hezar Jerib St., Isfahan University of Medical Sciences, Isfahan, Iran
Submission: October 12, 2019; Published: November 14, 2019

ISSN 2578-0190 Volume3 issues2
Abstract
Background: Chlamydia trachomatis (CT) has been considered as a major health problem worldwide given the high incidence of the infection, estimated to be 131 million new cases annually. The aim of the current study was to determine the prevalence of CT infection in female sex workers (FSWs) living in Isfahan, Iran and risks associated with its prevalence.
Methods: This was a cross-sectional study, which was conducted on 99 FSWs recruited from two drop-in centers (DIC) in Isfahan during 2012-2014. The attendees were recruited consecutively. Vaginal, rectal, saliva and urine samples were tested for CT using Real-time PCR. The demographic data were gathered by face to face interview.
Results: Prevalence of Chlamydia infection was 20.2% (20/99). The number of people who had more than one sexual partner in the CT-positive group was significantly higher than CT-negative (P= 0.042). The percentage of women whose duration of intimate relationship lasted more than three years was higher in the Chlamydia-positive population compared to Chlamydia-negative (70% vs 35.4%, p=0.005). Furthermore, Chlamydia-negative and Chlamydia-positive FSWs differed from one another in the history of imprisonment (11.4 % in Chlamydia-negative vs 35% in Chlamydia-positive group, p=0.017).
Conclusion: The prevalence of CT infection was high among FSWs. Our findings showed that prison experience, the number of sexual partners and duration of intimate relationship were statistically significant among those affected by chlamydia infection.
Keywords: Chlamydia trachomatis; Sexually transmitted infection; Sex workers
Introduction
Currently, Chlamydiosis is considered as one of the most frequent obligatory intracellular infections and a major health problem worldwide and WHO estimates that 131 million new cases of the disease annually occur. This disease is more prevalent amongst females and the majority of the cases are either asymptomatic or with mild clinical signs [1,2].
Asymptomatic nature of Chlamydiosis enhances its prevalence and the infected people are more likely to be afflicted by far-reaching implications such as pelvic inflammatory diseases, ectopic pregnancy, infertility, preterm labor and ophthalmic infections occurring in newborn infants. Accordingly, Chlamydia infection imposes high expenditure on community members as well as public health organizations [1,3].
Chlamydiosis more frequently occurs among those who have high-risk sexual behavior; hence, female sex workers (FSWs) hold the key to the understanding of the Chlamydia prevalence. A review study reported prevalence of Chlamydia ranged from 2.3% (among male prisoners) to 25.45% among women with abortion), 6.9% and 9% among FSWs resident at Tehran and Shiraz city respectively (25, 26). In this context, screening programs relying on precise and cost-effective approaches can efficiently control the burden of the disease [4,5].
Chlamydiosis was routinely diagnosed through cell culture or immunological approaches aiming at detection of specific antigens. These methods fail to meet appropriate sensitivity and need live bacteria and painful sampling. Molecular methods, which are based on polymerase chain reaction (PCR), such as Real Time PCR are considered as gold standards for Chlamydia infection diagnosis since they provide researchers with appropriate sensitivity and specificity [6,7].
The burden of Chlamydia infection widely differs from one community to another due to various cultural behaviors [6,8]. To the best of our knowledge, there is a little information regarding the prevalence of Chlamydia infection and to understand characteristics associated with its prevalence amongst sexually active women inhabiting in metropolitan areas of developing countries such as Iran. In the present study, we aimed to evaluate the prevalence of Chlamydia trachomatis (C. trachomatis) in FSWs living in Isfahan, Iran. Knowledge about prevalence of the disease in these women can help us screen and provide care for the patients and prevent its spread through the community. In Iran there seems to be a growing number of women who enter sex work at younger ages and growing number of men as clients for these women, but there is insufficient data about prevalence of STIs including CT infection, thus no screening is done in this field and there is risk for spreading the infection from asymptomatic individuals. Since it could be transmitted among sexual partners and spread through community and there is little data in this field in Iran. With knowledge about CT prevalence among FSWs we can screen and provide care for the infected individuals and prevent the spread of the disease in community. And knowing the factors associated with CT infection in this group of patients can be a guide to screen the most likely to be infected.
Methods
Study design
This was a cross-sectional study implemented in drop-in centers (DICs) located in Isfahan from 2012 to 2014. All parts of the study protocol were approved by Ethical Committee of Isfahan University of Medical Sciences (IUMS), Isfahan, Iran (project number: 291199). Taking part in the study was completely voluntarily and did not influence the quality of health services provided for FSWs.
Sampling and sample size
We calculated a required sample size of 100 individuals according to the prevalence of Chlamydiosis as 0.16 amongst FSWs (alpha level = 0.05, and precision ≈ 0.072) [9]. Sampling was carried out through non‐probability convenience sampling approach. Inclusion criteria were women with (a) age of 18 years or older, (b) sexual intercourse (vaginal, anal and/or oral) for payment in money in the last six months (as measured by self-administered questionnaire), (c) lack of antibiotic therapy during the past month. Those who did not fulfill specimen requirement were excluded.
Data collection
The study aim and procedures were clearly described for all of the participants meeting our inclusion criteria. We assured the potential participants that the study was anonymous. Afterward, informed consent was obtained from each participant. Demographic data, the number of sexual partners, history of drug abuse and alcohol consumption, duration of the intimate relationship, and record of imprisonment were logged by a trained female social worker through face to face interview.
Sample collection and laboratory analysis
Cervix investigation implemented through speculum in every patient preceded genital sampling. Genital sampling was taken through sterile cotton swab rolled within the endocervix for 10-30 seconds. We did not use antiseptics in the sampling procedure, nor did our samplings lead to bleeding. All of the swabs were collected in transport tubes containing 1.2ml of sterile 1X phosphate buffered saline (PBS, Cyto Matin Gen Company). Furthermore, a first-void urine sample (30ml of urine) was collected in a urine collection cup for every participant. These samples were kept at + 4ºC in a period of time, which did not exceed 72 hours. Extra-genital samples were provided from anorectal or pharyngeal secretion from all those who had anal and (or) oral sexual contacts regardless of sympyoms. Afterward, all of the samples were transported to the laboratory of Infectious Disease Research Institute, IUMS.
To assess Chlamydia infection, Real Time PCR was performed. Extracted DNA of C. trachomatis and sterile DNA/RNA free water were used as positive and negative controls, respectively. All of the primers and Taqman probes were specified for trp gene of C. trachomatis sequence, which was available in Genebank (http://www.ncbi.nlm.nih.gov). The sequences of primers were as follow: 5′-TTCAGTTGGGCCAGATCATG-3′ (F), 5′-CTCTTCATCGGTGGCTAATGTATAAA-3′(R). The sequence of Taqman probe was 5′-(FAM)-AGGCTCGTCCTGACTCATGCATTTCG-(TAMRA)-3′]. DNA of the collected samples was prepared using a high pure nucleic acid extraction kit (Roche, Germany), according to the manufacturer instructions. The amplification was carried out in a final volume of 50μl, including 2mM MgCl2, 1.5 U Taq DNA polymerase (Fermentas, USA), 1X PCR buffer, 0.35 μM primers, and 1μl DNA template. The conditions for PCR amplification were as follow: 94 °C for 5min, followed by 40 cycles of 94 °C for 15 s and 58 °C for 40s [10].
Statistical analysis
Independent samples t-test, Chi-square test, univariate and multiple logistic regression were applied to evaluate effects of age, marital status, education, number of sexual partners, duration of intimate relationship, condom usage, history of imprisonment,drug abuse and drinking alcohol (as asked by questionnaire)on the risk of Chlamydia infection in FSWs. Data analyses were performed by STATA 14 (StataCorp. 2015. Stata Statistical Software: Release 14. College Station, TX: StataCorp LP) at 5% significance level.
Results
A total of 99 Out of 104 FSWs met our inclusion criteria participated in the current study. The mean age of the participants was 35.14 years and about 87% of them were literate. Five percent of the infected individuals aged less than 25 years and nearly half of them were married (50.5%). About 16% of the FSWs had a history of imprisonment. In addition, 55% of the participants were opium addicted (as asked by questionnaire) and half of the FSWs were alcohol consumer (as asked by questionnaire).
Prevalence of Chlamydia infection was 20.2% (20/99). There were 15 FSWs being positive in urine samples and there was one participant, who was positive for urine and cervical sample. In addition, one rectal sample and three cervical ones were positive for C. trachomatis. There was no positive result in pharyngeal samples. The prevalence of Chlamydia infection for the genital and anorectal sites were 19.2% and 1% respectively. Results of the present study showed that age, marital status, education, drug abuse and drinking alcohol did not significantly differ between Chlamydia-infected and Chlamydia-noninfected individuals. The frequency of women having more than two sexual partners in a week differed between Chlamydia-infected (34.9%) and Chlamydia-noninfected individuals (65.1%) (p=0.004). There was a significant difference between Chlamydia-positive (33.3%) and Chlamydia-negative population (66.7%) between women whose duration of intimate relationship lasted more than three years (p=0.005). Furthermore, the percentage of women who had a history of imprisonment (43.7%) were significantly higher in the Chlamydia-positive group, compared with Chlamydia-negative one (14.8%, p=0.014) (Table 1).
Logistic regression analysis results showed that the crude odds of having a chlamydia infection were 4.04 times greater for those having more than two sexual partners (OR=5.04, 95% CI: 1.65-15.36); 3.25 times greater for more than 3 years duration of having sex (OR=4.25, 95% CI: 1.33-14.84) and 3.47 times greater for those with history of imprisonment (OR=4.47, 95% CI: 1.40-14.30). However, when the logistic regression model adjusted for age marital status, addiction and drinking alcohol, only number of sexual partners and history of imprisonment stayed statistically significant (Table 1).
Table 1: Descriptive characteristics of FSW & Odds Ratio (OR) of Chlamydia infection for each character.
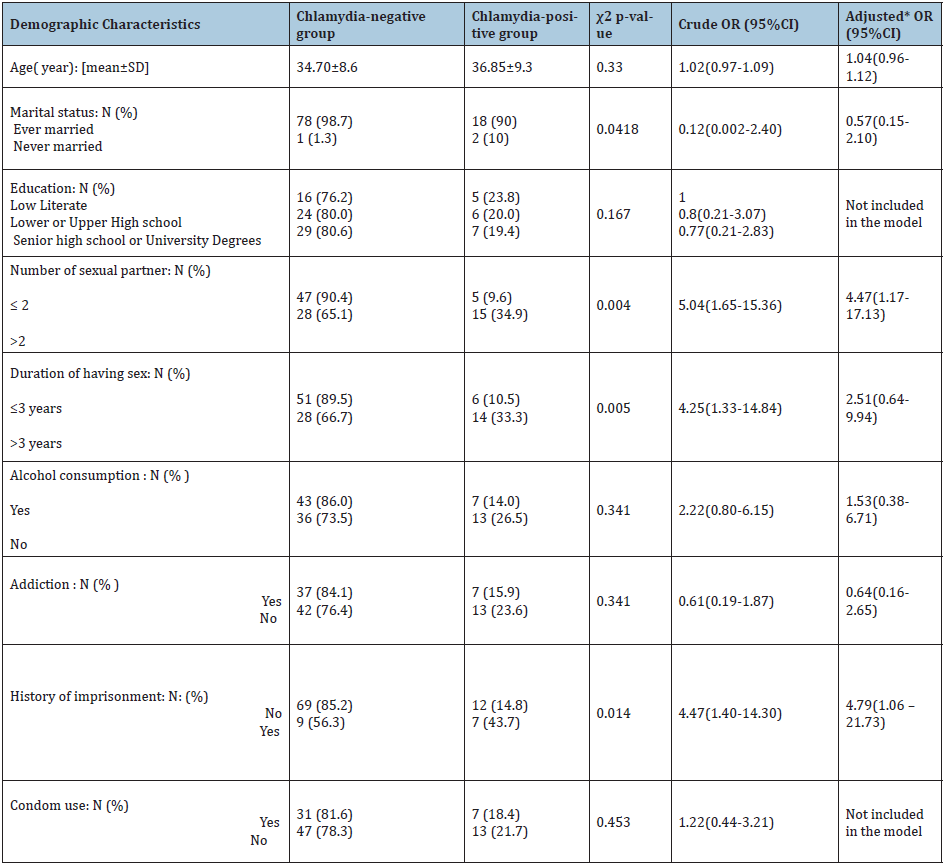
* Multiple logistic regression included age, marital status, duration of having sex, number of sexual partners, history of imprisonment, addiction and drinking alcohol.
Discussion
This study was conducted in Isfahan, where located at the main north–south and east–west routes crossing Iran (about 340 kilometers south of Tehran, capital city) with a different culture (religious city) on a specific population, FSWs. Sex work is illegal in Iran and FSWs are not organized. There is no specific place for sex business, therefore, currently, neither any screening plan nor official statistics on FSWs, but seems increasing. Our finding indicated that prevalence of Chlamydia infection amongst FSWs living in Isfahan was 20.2% and three characteristics including number of sex partner, duration of intimate relationship, and history of imprisonment significantly associated with Chlamydia infection.
Previous epidemiological studies on different study populations were undertaken in developing countries documented the various prevalence of Chlamydia infection ranging from 2% to 16.1% [6,9,26] that can be attributed to the fact that FSWs have more sexual partners than other women. For example, the prevalence of Chlamydia infection in females aged 17-35 years living in Kashan (Isfahan Province, Iran) was 2.4% [6], 6.9% in Tehran and Bandar Abbas [25], 9% in Shiraz among FSWs [26] and 25.45% among women with abortion [25].
The higher prevalence of Chlamydia infection among FSWs found in the current study could be either attributable to our study population (FSWs) (in comparison with Kashan [6] where general population was studied) or place of residents with different cultures (Tehran & BadarAbbass and Shiraz against Isfahan). In fact, we recruited vulnerable women referred to DICs; hence, in comparison with the general population or individuals reporting sexual assaults, a further prevalence of sexually transmittable infections (STIs) could be expected.
In two cities of India (Hyderabad and Mumbai), the prevalence of Chlamydia infection amongst FSWs was 16.1% [9]. Remis et al. [11] in 2014 showed that 14.7% of FSWs living in Shanghai (China) were infected with Chlamydiosis. By comparison with similar studies carried out amongst FSWs, our results indicated a higher prevalence of Chlamydia infection, which may be attributed to two following reasons. First, in India, the exchange of sexual services for money is legal if carried out in the private residence of a prostitute or others [27].
Although prostitution is illegal in China, there are common sights for sex workers in cities and towns of all sizes and designated STD clinics for health screening of FSWs [28]. However, no such a system in Iran. Second, it is, perhaps, attributable to the higher age of our study participants (35.4 years). In fact, FSWs were more likely to be affected by STIs when they were getting older. Accordingly, a different study showed that screening programs aiming at women aged 25 years and younger could just recognize 28% of Chlamydia infection, whereas screening of women aged up to 30 years could recognize 83% of the infected cases. As such, an extension of Chlamydia screening which is not limited to women aged 25 years and younger could be highly beneficial to identify more infected individuals [12].
In comparison, our study indicated a lower prevalence of extra-genital Chlamydia infection: 1% in anorectal and 0% in oropharyngeal samples, perhaps, due to small sample size. However, Chlamydia prevalence in anorectal and oropharyngeal specimens of French individuals being sexual assault victims, was 1.9% and 8.7% respectively [3]. Variation of extra-genital Chlamydia prevalence occurring in different communities might be induced by different factors comprising the geographic area, and cultural background [13] (as may be the case in other sexually transmitted disease like HIV infection). Total lower prevalence of oropharyngeal Chlamydia infection and fewer oral and anal sexual contacts among this studies’ participants reported in table 2. Furthermore, the age of the participants might affect the prevalence of extra-genital Chlamydia infection. In fact, participants in our study were relatively old (mean age: 35.14 years) whilst younger women are more dominantly considered as sexual assault victims. That is why we found a low prevalence of extra-genital Chlamydia infection [3].
We assessed the association of Chlamydia infection with demographic variables. We found that the prevalence of Chlamydia infection in individuals aged<25 years (8.3%) differed from those reported in Shanghai (19.6%). Higher prevalence of Chlamydia infection in Shanghai probably reflects a substantial increase of commercial sex workers living in large urban areas of China [11].
In the current study, Chlamydia infection was significantly greater in females experiencing greater than 3 years of sexual intercourse the crude odds of having a chlamydia infection were 3.25 times greater for FSWs with more than 3 years duration of having sex. These findings could be considered as an expected result since more years of sexual intercourse associated with not only higher numbers of sexual partners but also riskier behaviors such as not using condoms, opium addiction and drinking alcohol that prone the sex workers to unprotected sexual contacts (Table 2).
Table 2: Chlamydia infection prevalence by type of sexual contacts.
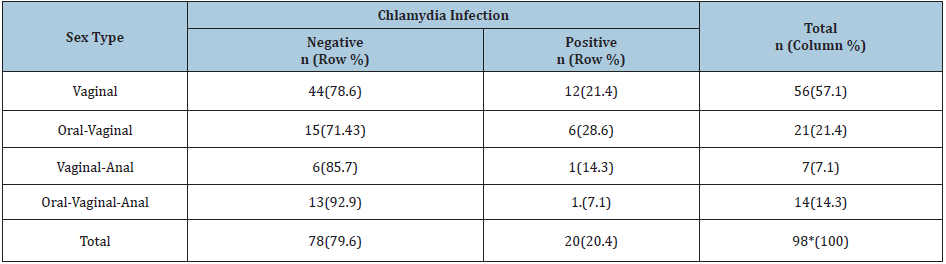
* One person did not answer her type of sexual contact.
Our study and a study in Saudi Arabia, showed that neither marital status nor educational level associated with Chlamydia infection [14]. However, Kwankwo et al. [15] authenticated that, by comparison to the single women, Chlamydia infection was more prevalent amongst married or divorced females. A plausible explanation was that Chlamydia genotype differing between various communities took responsibility for the above-mentioned discrepancy. In this context, a molecular epidemiological study documented a genotype-based association between C. trachomatis infection and marital status of patients [16].
The current study showed that the
odds of having a chlamydia infection were 4.04 times greater for those having more than two sexual partners Association between the number of sexual partners and the prevalence of Chlamydia infection was shown in some of the previous studies [17]. For example, in a study conducted in Sri Lanka, the risk of Chlamydia infection rose by three times in participants who had more than one sexual partner [18]. The effect of numerous sexual partners was attributable to asymptomatic nature of Chlamydia infection. As such, the likelihood of the disease transmission would dramatically rise according to the numbers of sexual partner [19].
Our results indicated that Chlamydia infection did not significantly associate with alcohol consumption or drug abuse. Consistently, a multicentral study, which was carried out in Latin America, Africa, and Eastern Europe, demonstrated that Chlamydia infection in the women did not associate with alcohol consumption or drug abuse [20]. Conversely, a different study, which was implemented amongst 6544 Swedish youth aged 15-24 years, authenticated that, in comparison with the participants drinking 2 times per week or less, the prevalence of Chlamydia infection increased when the women drank further [21]. The inconsistency found between our results and another could be attributable to sample size varying in different studies [21].
Our findings revealed that condom use did not have a significant association with Chlamydia infection. In a multicenter study, which was carried out in 11 health centers located in Benin, Burkina Faso, Ghana, Guinea, and Mali, there was not any significant association between condom usage and Chlamydia infection [22]. However, vast bodies of studies well documented the protective role of condom use against sexually transmitted diseases [23]. Noticeably, our study did not concern whether the participants consistently use condoms or not; hence, inconsistent condom use might affect our results [24]. Chlamydia trachomatis prevalence in FSWs was significantly associated with imprisonment; we believe this could be due to interaction between imprisonment and number of partners which increased for FSWs who had history of imprisonment.
Given the observational nature of this study, there are important limitations that must be highlighted as a result of small sample size and the convenience sampling and recruiting from drop-in centers. The discussion section does highlight that the recruitment strategy used may have resulted in selection bias, (biasing the results to a higher chlamydia prevalence). However, there was no mention of who may not have been captured by this study. We recommend other investigators to refine our estimates and provide more information for health policy makers to plan a systematic screening programs in Iran.
Acknowledgement
The authors of the current manuscript appreciate vice chancellor for research at Isfahan University of Medical Sciences and Isfahan Province Health Center as well as all the participants who so kindly agreed to contribute toward this research.
References
- Piñeiro L, Lekuona A, Cilla G, Lasa I, Martinez Gallardo LP, et al. (2016) Prevalence of Chlamydia trachomatis infection in parturient women in Gipuzkoa, Northern Spain. Springerplus 5(1): 1-5.
- Woodhall SC, Soldan K, Sonnenberg P, Mercer CH, Clifton S, et al. (2016) Is chlamydia screening and testing in Britain reaching young adults at risk of infection? Findings from the third National Survey of Sexual Attitudes and Lifestyles (Natsal-3). Sex Transm Infect 92(3): 218-227.
- Jauréguy F, Chariot P, Vessières A, Picard B (2016) Prevalence of Chlamydia trachomatis and Neisseria gonorrhoeae infections detected by real-time PCR among individuals reporting sexual assaults in the Paris, France area. Forensic Sci Int 266: 130-133.
- Vall-Mayans M, Villa M, Saravanya M, Loureiro E, Merono M, et al. (2007) Sexually transmitted Chlamydia trachomatis, Neisseria gonorrhoeae, and HIV-1 infections in two at-risk populations in Barcelona: female street prostitutes and STI clinic attendees. Int J Infect Dis 11(2): 115-122.
- Wong HT, Lee KC, Chan DP (2015) Community-based sexually transmitted infection screening and increased detection of pharyngeal and urogenital Chlamydia trachomatis and Neisseria gonorrhoeae infections in female sex workers in Hong Kong. Sexually transmitted diseases. 42(4): 185-191.
- Afrasiabi S, Moniri R, Samimi M, Khorshidi A, Mousavi SG (2015) The Prevalence of Endocervical Chlamydia trachomatis Infection Among Young Females in Kashan, Iran. Jundishapur J Microbiol 8(4): e15576.
- Fernández G, Martró E, González V, Saludes V, Bascuñana E, et al. (2015) Usefulness of a novel multiplex real-time PCR assay for the diagnosis of sexually-transmitted infections. Enferm Infecc Microbiol Clin 34(8): 471-476.
- Kassaian N, Ataei B, Yaran M, Babak A, Shoaei P (2011) Hepatitis B and C among women with illegal social behavior in Isfahan, Iran: Seroprevalence and associated factors. Hepat Mon 11(5): 368-371.
- Das A, Prabhakar P, Narayanan P, Neilsen G, Wi T, et al. (2011) Prevalence and assessment of clinical management of sexually transmitted infections among female sex workers in two cities of India. Infect Dis Obstet Gynecol 2011: 494769.
- Wei Hb, Zou Sx, Yang Xl, Yang Dq, Chen Xd (2012) Development of multiplex real-time quantitative PCR for simultaneous detection of Chlamydia trachomatis and Ureaplasma parvum. Clin Biochem 45(9): 663-667.
- Remis R, Kang L, Calzavara L, Pan Q, Liu J, et al. (2015) Prevalence and correlates of HIV infection and sexually transmitted infections in female sex workers (FSWs) in Shanghai, China. Epidemiol Infect 143(2): 258-266.
- Paukku M, Kilpikari R, Puolakkainen M, Oksanen H, Apter D, et al. (2003) Criteria for selective screening for Chlamydia trachomatis. Sex Transm Dis 30(2): 120-123.
- Zhang L, Chow EP, Su S, Yiu WL, Zhang X, et al. (2015) A systematic review and meta-analysis of the prevalence, trends, and geographical distribution of HIV among Chinese female sex workers (2000-2011): implications for preventing sexually transmitted HIV. Int J Infect Dis 39: 76-86.
- Fageeh W, Badawood S, Al Thagafi H, Yasir M, Azhar E, et al. (2014) Chlamydia trachomatis infection among female inmates at Briman prison in Saudi Arabia. BMC Public Health 14(1): 267.
- Nwankwo E, Magaji N (2014) Prevalence of Chlamydia trachomatis infection among patients attending infertility and sexually transmitted diseases clinic (STD) in Kano, North Western Nigeria. Afr Health Sci 14(3): 672-678.
- Zhang JJ, Zhao GL, Wang F, Hong FC, Luo ZZ, et al. (2012) Molecular epidemiology of genital Chlamydia trachomatis infection in Shenzhen, China. Sex Transm Infect 88(4): 272-277.
- Pépin J, Deslandes S, Khonde N, Kintin D, Diakité S, et al. (2004) Low prevalence of cervical infections in women with vaginal discharge in west Africa: implications for syndromic management. Sex Transm Infect 80(3): 230-235.
- Mangalika G, Cankanamge SK, Priyadarshana D, Shamini P, Sujatha M, et al. (2014) Prevalence of Chlamydia trachomatis in women attending sexually transmitted disease clinics in the Colombo district, Sri Lanka. Indian J Pathol Microbiol 57(1): 55-60.
- Ahmadi MH, Mirsalehian A, Bahador A (2015) Prevalence of genital Chlamydia trachomatis in Iran: a systematic review and meta-analysis. Pathog Glob Health 109(6): 290-299.
- Folch C, Esteve A, Sanclemente C, Martró E, Lugo R, et al. (2008) Prevalence of human immunodeficiency virus, Chlamydia trachomatis, and Neisseria gonorrhoeae and risk factors for sexually transmitted infections among immigrant female sex workers in Catalonia, Spain. Sex Transm Dis 35(2): 178-183.
- Hammarström S, Tikkanen R, Stenqvist K (2015) Identification and risk assessment of Swedish youth at risk of chlamydia. Scand J Public Health 43(4): 399-407.
- Stanback J, Shelton JD (2008) Pelvic inflammatory disease attributable to the IUD: modeling risk in West Africa. Contraception 77(4): 227-229.
- Subramanian T, Ramakrishnan L, Aridoss S, Goswami P, Kanguswami B, et al. (2013) Increasing condom use and declining STI prevalence in high-risk MSM and TGs: evaluation of a large-scale prevention program in Tamil Nadu, India. BMC public health 13(1): 1.
- Crosby RA, DiClemente RJ, Wingood GM, Lang D, Harrington KF (2003) Value of consistent condom use: a study of sexually transmitted disease prevention among African American adolescent females. Am J Public Health 93(6): 901-902.
- Janghorban R, Azarkish F (2016) An overview on sexually transmitted infections in Iran. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 5(3): 585-595.
- Kazerooni PA, Motazedian N, Motamedifar M, et al. (2013) The prevalence of human immunodeficiency virus and sexually transmitted infections among female sex workers in Shiraz, South of Iran: by respondent-driven sampling. Int J STD AIDS 25(2):155-161.
- https://indiacode.nic.in/bitstream/123456789/6818/1/ind93633.pdf
- (2009) "2008 Human Rights Report: China (includes Tibet, Hong Kong, and Macau)". United States Department of State.
© 2019 Somayeh Haghighipour. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






