- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Effect of Temperature and Growth Media on Mycelium Growth of Pleurotus Ostreatus and Ganoderma Lucidum Strains
Ian Fletcher*, Aisha Freer, Ash Ahmed and Pauline Fitzgerald
School of Art, Architecture & Design, England
*Corresponding author:Ian Fletcher, Senior Lecturer (0.5FTE), School of Art, Architecture & Design, B502 Broadcasting Place, England
Submission: June 26, 2019; Published: July 09, 2019

ISSN 2578-0190 Volume 2 Issue 5
Abstract
The aims of the study are to identify an effective and versatile fungal strain for bioengineering mycelium composites. The influence of temperature and four different growth media on mycelium growth of two white rot fungi, Pleurotus ostreatus (Winter Oyster) and Ganoderma lucidum (Reishi) were investigated in laboratory conditions. The results of the experiment indicated that potato dextrose agar (PDA) was the most suitable growth media for mycelium growth of fungal strains, P. ostreatus and G. lucidum. However, P. ostreatus was the better performing strain, with highest mean mycelium growth of 23.28cm, compared to G. lucidum at 9.03cm on PDA after 12 days of inoculation. Potato dextrose agar (23.28cm) and potato dextrose agar supplemented with yeast extract (14.74cm) were more favorable for mean radial mycelium growth of P. ostreatus, followed by sabouraud dextrose agar (9.85cm) and iron sulphite agar (8.35cm). The fungal strain, P. ostreatus obtained improved mycelium morphology on potato dextrose agar (PDA) supplemented with yeast extract and obtained cottony textured mycelium with good density and growth on potato dextrose agar (PDA) and sabouraud dextrose agar (SDA). Iron sulphite agar (ISA) was least favourable growth media for mean radial mycelium growth and mycelium morphology. Fungal strain, G. lucidum only mycelium growth was obtained on potato dextrose agar (PDA) as a result of this study, was the least favourable fungal strain studied. Optimal temperature for mycelium growth for both fungal strains, P. ostreatus and G. lucidum was obtained at 22 °C.
Keywords: Mycelium growth; Temperature; Growth media; Pleurotus ostreatus; Ganoderma lucidum
Abbreviations: PDA: Potato Dextrose Agar; SDA: Sabouraud Dextrose Agar; ISA: Iron Sulpice Agar
Introduction
In the last decade fungal mycelium has gained much attention from academics and commercial enterprises due to its ability to upcycle agricultural and industrial wastes into sustainable composite materials. The process utilizes a natural, low-energy manufacturing process able to sequester carbon and create useful alternative materials [1]. Mycelium composites comprise of networks of filamentous hyphae which can be converted into economically viable and environmentally friendly materials utilizing biological growth rather than expensive energy intensive manufacturing processes Jones et al. [2]. There are a few fungal species described in literature which can be used for composite manufacturing. The most commonly used fungal species are Pleurotus ostreatus and Ganoderma lucidum[2-6]. P. ostreatus and G. lucidum are two edible, fast-growing, commonly available mushrooms belonging to the fungal group, Basidiomycota. Basidiomycota (colloquially basidiomycetes) are a large group of fungi with over 30, 000 species [7]. One of the main characteristics of Basidiomycota is its ability to obtain necessary nutrition for its growth and lifecycle. With photosynthetic pigments absent, the fungal group have a heterotrophic mode of nutrition, which obtain nutrients by extracellular digestion due to the activity of secreted enzymes, followed by the absorption of solubilized breakdown products. This combination of extracellular digestion and absorption can be seen as the ultimate determinant of fungal lifecycle [7]. Therefore, colonization of an ideal food source is essential for growth of a network system of branching hyphae, which collectively makes up the mycelium. According to Jones et al. [8], hyphal characteristics vary significantly by species, which in turn determines their application .The most influential growth performance factors are saprotrophic fungi, in conjunction with environmental conditions and chemical nutrition. The main saprotrophic fungi are P. ostreatus and G. lucidum, which can play a vital role in upcycling a range of agricultural wastes for the production of mycelium composites. The mechanical performance of these materials varies significantly and are governed by hyphal architecture, cell wall composition, composite constituents and growth kinetics, which in turn are influenced by inherent and exogenous factors, such as temperature and nutritional conditions Jones et al. [2] . However, the main factors that affect mycelium growth for processing composite materials are temperature, growth media, carbon and nitrogen sources, and lignocellulosic substrate sources. As yet, these variables are not well defined in the literature. In this study P. ostreatus (strain M2175) and G. lucidum (strain M9720) are studied to determine optimal temperature and growth conditions necessary for optimal mycelium growth performance.
Material and Experimental Methodology
Fungal cultures and materials
Fungal species were obtained from Mycelia (Belgium) as a mycelial mass on digested wheat grain sealed in plastic bags with filter patches. The growth media was potato dextrose agar, sabouraud dextrose agar, iron sulphite agar and potato dextrose agar supplemented with yeast extract. These were obtained from the biomedical research laboratory in the School of Clinical and Applied Sciences at Leeds Beckett University.
Effect of temperature and growth media on mycelium growth
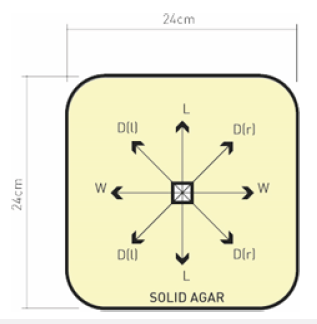
The effect of temperature and growth media on mycelium growth of two white-rot fungi, P. ostreatus and G. lucidum were studied. Mycelium starter cultures were inoculated onto agar plates containing a mixture of sabouraud dextrose agar (150ml) and ground wheat straw (5g). The growth medium was autoclaved at 121 °C for 15 minutes (at a pressure of 1.1kg/cm2) and then inoculated with mycelia from the stock culture. The inoculated plates were incubated at room temperature for a duration of 7 days under dark conditions. Growth media used in this study were potato dextrose agar (PDA) and sabouraud dextrose agar (SDA). Growth media were prepared by dissolving 39g of PDA in 1000ml distilled water and 65g of SDA in 1000ml distilled water in 2L glass bottles (Schott Duran). Bottles were placed on a magnetic stirrer until agar was fully dissolved and then autoclaved at 121 °C for 15 minutes. After sterilization, 150ml of PDA and SDA were each poured into square petri dishes measuring 24cm(L)x24cm(W)x2.5cm(H) and allowed to solidify. Under aseptic conditions in a laminar flow chamber (Esco Lab culture) inoculum disks measuring 1x1cm square was cut from starter culture using sterile knives. New sterile knives were used for each strain and a single inoculum disk was placed in the center of each agar plate. Dishes were covered with lids and incubated in darkness in an incubator (Genlab) at 22 °C, 30 °C and 37 °C for each fungal strain. Mean radial mycelium growth in centimeters were measured from center of inoculum disk to tip of hypha in four directions, (Figure 1). This was completed each day until square petri dishes were fully covered by mycelium. Each experiment was replicated 3 times containing a single inoculum.
Effect of different growth media on mycelium growth
The effect of different growth media on mycelium growth were studied. Growth media used in this study were potato dextrose agar (PDA) supplement with yeast extract (PDYA) and Iron sulphite agar (ISA). Growth media were prepared by dissolving 39g of potato dextrose agar and 20g of yeast extract in 1000ml of distilled water and 40.5g of ISA in 1000ml of distilled water in 2L laboratory glass bottles (Schott Duran). Bottles were placed on a magnetic stirrer until the agar was fully dissolved and autoclaved at 121 °C for 15 minutes. After sterilization, 150ml of PDYA and ISA were each poured into square petri dishes (same as above) and allowed to solidify. Under aseptic conditions in a laminar flow chamber (Esco Lab culture) inoculum disks of P. ostreatus, measuring 1x1cm square was cut using sterile knives and placed in the center of each agar plate. Dishes were covered with lids and incubated in darkness in an incubator (Gen Labs) at optimal temperature of 22 °C for the duration of 12 days. Mean radial mycelium growth was performed as described earlier. Each experiment was replicated 3 times containing a single inoculum.
Figure 1:Mycelium growth measured (cm) as mean hyphal growth on agar solid media plates, where W, L, DL and DR are mean mycelium growth.
Result and Discussion
Effect of temperature and growth media on mycelium growth
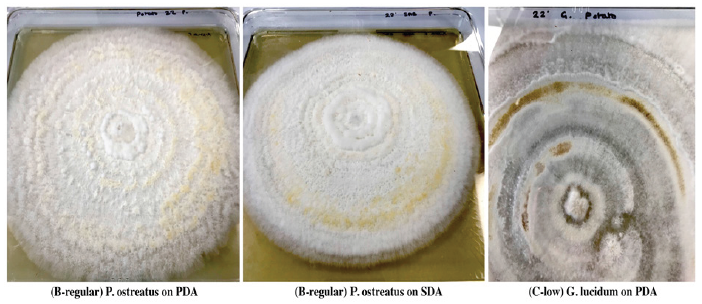
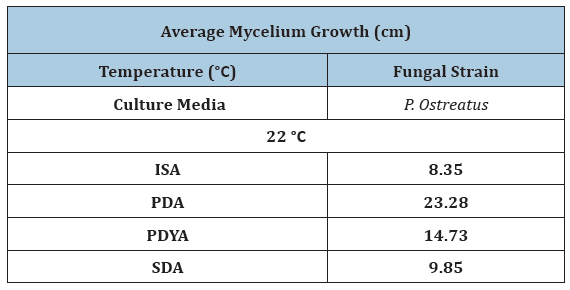
Temperature is one of the most important environmental factors for mycelium growth and growth occurs over a wide range of temperature. To determine optimal temperature for mycelium growth of P. ostreatus and G. lucidum it was necessary to furnish compounds required for its growth and life process. In this study, fungal strains were cultivated on PDA and SDA growth media at temperatures of 22 °C, 30 °C and 37 °C in the laboratory. Results in Table 1 show that mean mycelium growth of P. ostreatus and G. lucidum were both directly influenced by temperature and growth media (P≤0.05). Mycelium growth of P. ostreatus was significantly better than that of G. lucidum on PDA (23.28cm) and SDA (9.85cm) at 22 °C and 30 °C on PDA (5.43cm) and SDA (4.43cm) after 12 days of inoculation. P. ostreatus grew mycelium in a wider range of temperature 22 °C ~30 °C on both PDA and SDA. Mycelium growth of P. ostreatus and G. lucidum grew optimally at a temperature of 22 °C. These findings were similar to Neelam et al. [9], who reported optimum temperature for P. ostreatus in a range of 25 °C~30 °C. However, contradictory to observations by [10,11], who both reported an optimal temperature of 28°C for P. ostreatus. These results inferred that the strains used in this study were different from those reported. Nwokoye et al. [11], described three different strains of P. ostreatus. The high temperature strain (25°C-30°C), medium temperature strain (16 °C-22 °C) and low temperature strain (12 °C-15 °C). Suggesting that the strain used in this study belonged to the medium temperature strain. At optimum temperature, P. ostreatus was the better performing strain with a maximum mean mycelium growth of 23.28cm (PDA) and 9.85cm (SDA) after 12 days of inoculation. Mycelium growth of P. ostreatus was somewhat restricted at 30 ℃ on PDA (5.43cm) and SDA (4.43cm), while no growth was recorded for fungal strain G. lucidum. According to Jayasinghe et al. [12], exposure to high temperature can cause denaturation and inactivation of important enzymes which catalyze metabolic processes of tested mushroom strains. This was observed for both fungal strains at 37 °C, where no mycelium growth was obtained on either PDA or SDA. Results in (Figure 2) show PDA was the most suitable growth media for mean mycelium growth of both P. ostreatus (23.28cm) and G. lucidum (9.03cm) after 12 days of inoculation. The maximum mean mycelium growth of P. ostreatus on SDA was recorded at 9.85cm on day 12. Mean mycelium growth of P. ostreatus on PDA performed better than those on SDA (P≤0.05). There was significant difference in growth on day 4 onwards for P. ostreatus on PDA (6.13cm) compared to SDA (3.90cm). These findings were similar to results reported [13-14], whose studies both reported PDA as the most suitable media for culturing P. ostreatus in laboratory conditions. Mycelium morphologies were classified as described by Mendoza G et al. [15]. P. ostreatus produced cottony-textured, regular density and regular mycelium growth on PDA and SDA, while G. lucidum produced floccose-textured, low density and scarce mycelium growth on PDA (Figure 3). Yang et al. [16], reported P. ostreatus as a widely cultivated fungal strain due to its ability to grow in a range of wide temperature and utilize a variety of nutritional conditions. As a result of this, fungal strain P. ostreatus became the preferred strain to study effect of different growth media on mycelium growth.
Table 1:Effect of various temperatures and growth media on mycelium growth of P. Ostreatus and G. lucidum grown on potato dextrose agar (PDA) and sabouraud dextrose agar (SDA).
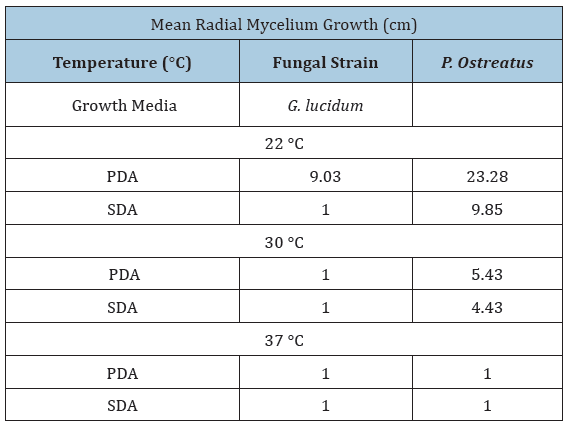
Figure 2:Mycelium Morphology of P. Ostreatus and G. lucidum on PDA and SDA growth media: (A-high) cottony with high density and abundant growth; (B-regular) cottony with regular density and regular growth and (C-low) floccose with low density and scarce growth.
Effect of different growth on mycelium growth
In this study the effect of different growth media was studied and compared to results from previous study to screen nutritional conditions for mycelium growth of P. ostreatus. The results indicated that PDA (23.28cm) and PDYA (14.73cm) as the most suitable growth media, while SDA (9.85cm) and ISA (8.35cm) were least favored for mycelium growth of P. ostreatus, Table 2. These results were similar to [13,14], both reported potato dextrose agar (PDA) as the most suitable media for optimal mycelium growth of P. ostreatus. [9], comparative studies on growth parameters and physic-chemical analysis of P. ostreatus and P. Florida, reported dextrose as the most suitable carbon source for optimal mycelium yield. Dextrose, which is an isomer of glucose, can be transformed to glucose during metabolism Neelam et al. [9]. Nitrogen and carbohydrates are essential elements required by all fungi. Nitrogen is responsible for the synthesis of nitrogen containing compounds and carbohydrates play key roles as structural and storage compounds in cells Hoa et al. [10]. PDA supplemented with yeast extract significantly improved mycelium morphology; however, its restricted mycelium growth somewhat Figure 4. These findings were similar to studies by Neelam et al. [9], who reported growth media supplemented with yeast extract as a suitable organic source of nitrogen for improved mycelium growth of P. ostreatus. But contrary to [13], who reported growth media containing ammonium chloride (NH4Cl) as the best nitrogen source for mycelium yield of P. ostreatus. According to Hoa et al. [10], chemical composition of growth medium, especially nitrogen concentration is a mean of physiological control and regulation of microorganism metabolism. High concentration of nitrogen can lead to a too low carbon to nitrogen ratio (C:N), resulting in restricted mycelium growth of P. ostreatus. ISA yielded the worst mycelium growth rate and mycelium morphology in this study. This may be due to high concentration levels of available nitrogen in growth media [17]. Hoa et al. [10], reported restricted mycelium growth of P. ostreatus due to high concentration levels of nitrogen. These results of P. ostreatus (M2175) mycelium growth indicates that the fungal strain has specific growth conditions necessary for its growth and it’s also directly related to ratio of carbon to nitrogen ratio (C:N). The types and concentrations can greatly influence growth kinetics Ajdari et al. [18].
Figure 3:Effect of various temperatures and growth media on fungal strains P. Ostreatus and G. Lucidum.
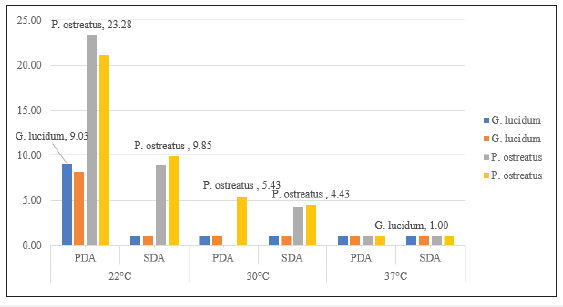
Figure 4:
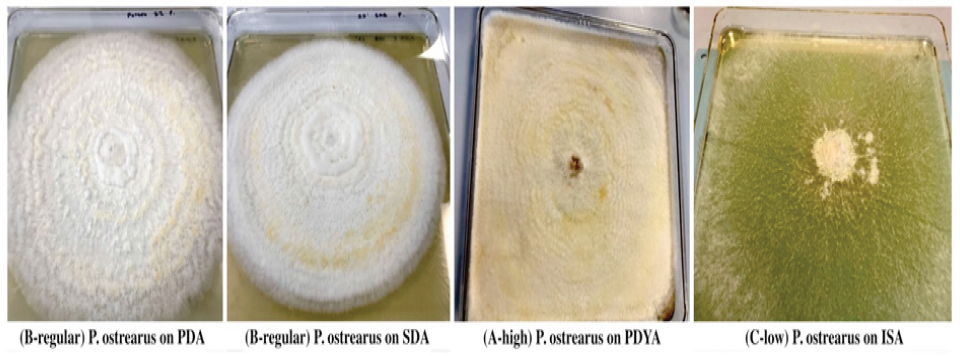
Table 2:Effect of four different growth media on mycelium growth of P. Ostreatus.
Summary
In summary, P. ostreatus supplemented with carbon and nitrogen source would be preferred strain for further studies on developing mycelium composites with wheat straw. It took a duration of 12 days for square petri dishes to be fully covered by mycelium growth. The fungal strain P. ostreatus obtained maximum mean growth on potato dextrose agar (23.28cm), followed by potato dextrose agar supplemented with yeast extract (14.73cm), sabouraud dextrose agar (9.85cm) and iron sulphite agar (8.35cm). This showed P. ostreatus to be a versatile fungal strain able to utilize various nutritional conditions but had specific growth requirements for optimum mycelium growth and morphology. The results of mycelium growth for P. ostreatus on different growth media, but significantly different (P<0.05), would suggest the macro-elements used in this study are required for growth, but within a tolerable limit. Potato dextrose agar was most suitable growth media for culturing P. ostreatus (23.28cm) and G. lucidum (9.03) in laboratory conditions. P. ostreatus produced best mycelium morphologies on potato dextrose agar supplemented with yeast extract (PDYA) followed by potato dextrose agar (PDA) and sabouraud dextrose agar (SDA). This showed mycelium morphology of P. ostreatus can be significantly improved with supplemented carbon and nitrogen sources grown at optimal temperature of 22 °C.
References
3. Haneef M, Ceseracciu L, Canale C, Bayer IS, Guerrero HJ. et al. (2017) Advanced materials from fungal mycelium: Fabrication and tuning of physical properties. Scientific Reports, pp. 1-11.
9. Neelam S, Chennupati S, Singh S (2013) Comparative studies on growth parameters and physio-chemical analysis of Pleurotus Ostreatus and Pleurotus Florida. Asian Journal of Plant Science & Research 3(1): 163-169.
11. Nwokoye AI, Kuforiji OO (2010) Studies on mycelial growth requirements of Pleurotus Ostreatus (Fr.) Singer. International Journal of Basic & Applied Sciences 10(02): 47-53.
17. Microbiology (1971) 86128 Sulfite iron agar (Iron Sulfite Agar). Microbiology.
© 2019 Ian Fletcher. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






