- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Growth Factors in the Human Body: A Conceptual Update
Estefania Alexandre1 and Debasish Bandyopadhyay1,2*
1Department of Chemistry, The University of Texas Rio Grande Valley, USA
2School of Earth Environment & Marine Sciences (SEEMS), The University of Texas Rio Grande Valley, USA
*Corresponding author:Debasish Bandyopadhyay, Department of Chemistry, and School of Earth Environment & Marine Sciences (SEEMS), The University of Texas Rio Grande Valley, 1201 West University Drive, Edinburg, Texas 78539, USA
Submission: May 09, 2019; Published: May 21, 2019

ISSN 2578-0190 Volume2 Issue5
Abstract
Growth factors are found in most of the organisms including insects, humans, plants, etc. The name ‘growth factors’ became known when the substances were active in stimulating the growth of cells and tissues. Growth factors are regulatory species (signaling molecules mainly proteins) that are generated by the body which function in binding to receptors on the cell surface and are required to stimulate the growth in living responsive cells by triggering cellular proliferation and/or cellular differentiation pathways, as well as by regulating gene transcription in the nucleus. They are quite versatile by promoting cell growth and act in a paracrine, retrocrine, autocrine, or juxtacrine signaling pathways. Cellular division is stimulated by growth factors in different cell types, while others can be specific to a cell type. Every growth factor binds to a specific receptor on the cellular surface. Cytokines are a family of growth factors that help in stimulating the movement of cells towards inflammatory sites, trauma, and infection. Abnormal regulation and production of growth factors and cytokines tends to cause various diseases that include, but are not limited to, cancer, diabetes, liver fibrosis, and bronchopulmonary dysplasia. A critical discussion with a focus on the diverse role and abundance of front-line growth factors in human body is presented. Bio-pharmacological relationships between growth factors with several diseases have also been incorporated in this article.
Keywords: Growth factors; Cell proliferation; Cellular differentiation; Cytokines; Fibroblast
Introduction
Growth factors were discovered when investigators studied biological substance effects on cells and tissues in cultures, identified an assembly of substances that were peptidehormone- like which were different from any hormones previously known. The similarity of growth factors with hormones is that some growth factors can be secreted into the blood stream, transporting them to their tissues. However, the production of hormones is strictly limited to glandular tissue. On the other hand, many types of tissues can produce different types of growth factors. An example of a class of signaling proteins that can act as growth factors are cytokines. They are used widely in cellular communication, in the function of the immune system, and in embryogenesis. In the past, growth factors and cytokines were thought to respectively serve as cell growth components and immunological response factors [1]. Most recently, research has stated that growth factors for example cytokines can have similar functions and can be used interchangeably. There are several different cellsignaling mechanisms are used to best suite its purpose. Paracrine signaling occurs between neighboring cells where the signals get quick responses and last only a short amount of time due to the degradation of the paracrine ligands [2,3]. In autocrine signaling, a cell signals itself through a component that synthesizes, leading to a biological response within the same cell. In endocrine signaling, growth factor and cytokine components are secreted into the blood and are then carried by blood and tissue fluids on to target cells where by subsequent responses are triggered [3]. Growth factors can also be divided into various families or super families based on structural and functional characteristics. An example of a major growth factor family is the transforming growth factor-β (TGF-β superfamily). TGF-β was originally characterized as a protein that was capable of inducing a transformed phenotype in non-neoplastic cells in culture. In TGF-β, all the family members have dimeric structures and their heterodimeric receptor complexes are consisted of type I and type II receptor subunits with serine/ threonine kinase domains. Within the family, complex interaction remains the same. Following ligand binding, the type I receptor is phosphorylated and stimulated by the type II receptor, which then activates a Smad-dependent signaling pathway, regulating gene transcription [3]. For research purposes, growth factors are used in cell models to study their functions, and their signaling pathways. Based on their roles, many growth factors in cytokines have also been utilized for preclinical and clinical applications. Recombinant human platelet-derived growth factor (PDGF) is an example of a growth factor, made by platelets in blood clots, and has been used to stimulate angiogenesis, migration, and mitosis in mesenchymal cells in preclinical studies. Although clinical applications of cytokines are considered as standard treatment for certain diseases but there are also side effects associated with this treatment. As an example, the administration of granulocyte-colony stimulating factor (G-CSF) has been reported to cause osteoporosis, acute coronary syndrome, and bone marrow necrosis. High quality growth factors must pass various quality control testing for purity, quantification, and activity. Purity tests, such as SDSPAGE can determine the possible contamination with non-specific proteins, and/or endotoxins that can trigger undesirable effects such as cell differentiation. Quantification is used to achieve product size and results. Commonly used protein quantification methods are Bradford colorimetric dye, UV-visible spectroscopy, and Densitometric comparison. Growth factor activity is tested through specific bioassays that determine the relative strength of the growth factor to a standard [1-3].
Growth Factors: Major types
There are various kinds of growth factors that exist, and several were initially isolated from animal tissues, including cattle and mice. Insulin-like growth factors are examples of these substances because they stimulate growth by growth hormone secretion from the pituitary gland; the epidermal growth factor, which stimulates the growth of epidermal cells; the nerve growth factor, which stimulates the growth of neuronal cells, and lastly, the plateletderived growth factor, which stimulates the growth of connective tissue cells and also muscle cells. Insulin-like growth factor I is also called somatostatin C, IGF-I. This hormone is widely discussed for possible performance enhancement and shares the common name “insulin” which is the medication used to treat type I diabetes [4]. The secretion of IGF-I is regulated by human growth hormone, which is produced by the pituitary gland. IGF-I is produced, secreted primarily from the liver. There are other peripheral tissues such as bone, that produces its own IGF-I. The reason behind the name of IGF-I is that it has insulin like subunit that binds to insulin receptors, but it only has 10% of the potency of regular insulin on glucose regulation. Insulin-like growth factor I (IGF-1) is an anabolic hormone, meaning it promotes growth. It includes skeletal muscle and it is not androgenic, as opposed to testosterone which indicates that it does not promote male sex characteristics such as facial hair, deep voice, etc. The side effects of IGF-I are low blood sugar, retinal edema, severe muscle pain, and bell’s palsy [1,4]. Figure 1 summarizes the relationship of IGF-1 with various cellular/physiological processes.
Figure 1:
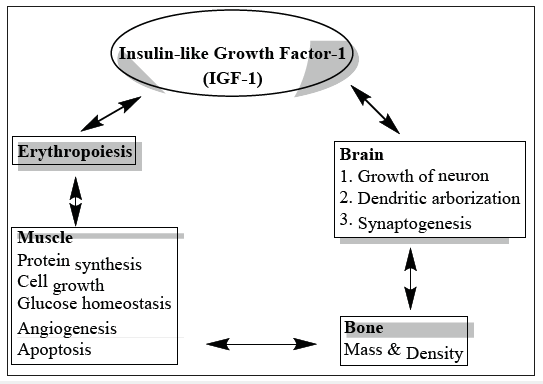
Growth factors play key roles in human body. The major primary effect is growth stimulation; it affects all tissues equally in the body, not just skeletal muscle, and occurs because it is released in the blood stream by the liver. Other functions include whole body protein synthesis, and inhibition of protein breakdown, low blood sugar, increased kidney filtration, enhanced bone mineralization, and more. Its function overlaps with human growth hormone (HGH). HGH is a hormone that is responsible for the production of growth factors and perform various functions in the human body including developmental and operational functions. After maturity approximately around age 20, the HGH that the body produces declines naturally and dramatically, but HGH is still necessary during adulthood in order for growth, cellular repair and regeneration to occur. However, HGH is not a good performance enhancer because a recent study found where the supplementation of growth hormone is no more effective at skeletal muscle growth than a placebo. The side effects of HGH include arthralgia, arthritis, enhanced heart, muscle weakness, high cholesterol, impaired glucose regulation and increased risk of type I diabetes, impotence, and increased fatigue [4]. As it has been mentioned earlier, growth factors act as typical signaling proteins and are also capable to regulate/contribute in several dreadful diseases like cancer, diabetes, HIV, cardiovascular diseases and so on. A brief account elaborating the influences of growth factors on these diseases are presented in the sequel.
Role of growth factors in Cancer
Growth factors frequently are involved in the production of resistance to therapeutic procedures, prolonging the role of polypeptide factors to precise late stages of tumor development and offering opportunities for cancer therapy. Studies done in the early 1950’s were one first occurring lines of evidence regarding cancer with soluble growth factors. The mechanisms that allow limb innervation in chick embryos, and transplanted a lump sarcoma found on a mouse onto an embryo were studied by observing thorough attraction of nerve fibers to the sarcoma lump. Later, a venom from a snake was identified and the murine submaxillary gland secreted a particularly active “nerve-stimulating factor”, that began with the isolation of the first growth factors: Nerve Growth Factor (NGF) and Epidermal Growth Factor (EGF). It was reported that cells that were infected by the ‘feline sarcoma virus’ lost their ability to bind EGF, leading to the isolation of a murine sarcoma of two “transforming growth factors”, TGF-α and TGF-β [5,6].
Cancer drugs are the focus of intense clinical investigation. Angiogenesis is mainly driven by 3 angiokinase pathways and is critical for diseases such as solid cancers, where new blood vessels deliver oxygen and nutrients to tumors, enabling tumor growth and metastasis. Blood vessels are composed of many cell types, that include endothelial cells, smooth muscle cells, and pericytes. Three signaling molecules that activate receptor tyrosine kinases on pericytes, vascular smooth muscle cells, and endothelial cells in order to initiate important proangiogenic signaling cascades are fibroblast growth factor (FGF), platelet-derived growth factor (PDGF), and vascular endothelial growth factor (VEGF). VEGF and FGF stimulate endothelial cell growth migration and survival, critical for new blood vessel formation. PDGF stimulates pericyte recruitment, that is needed for normal microvascular stability and function. PDGF is also identical to the RNA tumor virus called simian sarcoma virus. PDGF and FGF stimulate smooth muscle cell recruitment, critical for maturation and maintenance of blood vessels. All three growth factors are crucial to blood vessel formation maintenance and integrity [5,6].<./
VEGF is a homodimeric glycoprotein and a predominant angiogenesis pathway that is a target for anticancer therapy, making it the most important growth factor. The reason being is that its angiogenesis-promoting activity is at the level of the endothelial cell, and tumor penetration is less critical for VEGF inhibitors because it is compared with agents that directly target tumor cells. Tumors can activate alternative or escape mechanisms, and can upregulate FGFR, either spontaneously or during VEGF blockade. The result of the “angiogenic switch” is caused by the production of VEGF and other growth factors by the tumor, making it grow exponentially and leading to high interstitial pressure. Many biomarkers have been recognized as prognostic effectors and novel metastatic markers, targeting biomarkers such as growth factors and chemokines in gastric cancer (GC). In addition, tumors overexpress FGFR and PDGFR, representing a direct target for growths inhibiting FGFR and/or PDGFR by blocking all three angiokinase pathways, and making an approach to affect tumor growth by inhibiting critical steps in angiogenesis, increasing the effectiveness of chemotherapy and radiotherapy. Through several studies it was suggested that triple angiokinase inhibition may prevent further tumor growth and related tumor escape mechanisms. Bevacizumab is a humanized monoclonal drug that is used to treat several types of cancers and is directed at VEGF, making it the most advanced in clinical development by making VEGF a rational focus for anticancer therapy, showing promising results in clinical trials [6,7].
About 1.6% of the 22,000 functional genes in the human genome display intermittent somatic mutations in cancer. It should be considered that genes in the pathways are due to driver mutations not being able to interrupt on more than one gene in a pathway. Few gene families inside protein kinase descends that are placed downstream of growth factor receptors are frequently mutated in certain tumors. For example, pancreatic (RAS), melanomas (B-RAF), brain cancer (EGFR), and breast (ErbB-2/HER2). However, co-existence of such mutations and driver mutations that directly affect growth factor genes are very rare [8].
Prostate cancer (PCa) is the most common type of cancer in males. Recent studies suggest patients who have diabetes and take metformin (MF), have a lower chance of getting PCa. MF helps by lowering blood glucose levels, preventing the liver from making additional glucose. The growth factor, IGF-1 causes production and growth of PCa and MF has antineoplastic effects like adenosine monophosphate-activated protein kinase (AMPK), which is the suppression of androgen signaling pathways. Conveyance of metaanalysis proposes mortality benefit to patients who display PCa when taking MF, improved significantly [9].
Role of growth factors in Diabetes
Type 1 diabetic mellitus (T1DM) is known to adversely affect pubertal development and linear growth. Insulin-like growth factor-1 (IGF-I) is an extension of a growth hormone and seems to be associated in the earlier stages of the disease. Low levels of IGF may be linked to increased secretion of growth hormones in people with type 1 diabetes. IGF-1 is produced in the liver as an endocrine hormone and in target tissues. The production IGF-1 has the highest rates during pubertal growth spurt and have the lowest levels in infancy and old age. The growth hormone concentration in people who have diabetes are generally 3 to 4 times higher than people who do not have the disease. Abnormal levels of IGF and growth hormones may also increase the chances and complications of diabetes. Growth hormone has a pulsating secretion with agedependent concentrations that are characterized by mediocre secretion in the pre-pubertal period, a rise at puberty, and a reduction in old age. Results of clinical trials evaluating IGF-1in both type 1 and type 2 diabetes showed a great result in reduction of hemoglobin A1C levels, and daily insulin consumption. Insulinlike growth factor-binding protein (IGFBP-3) is an abundant protein that is encoded by the IGFBP3 gene and is the most important ICFP circulating during early age and is growth hormone dependent. IGFBP-3 carries IGFs to the target tissues and prolongs the halflife of IGFs, making a ternary complex with acid labile subunit (ALS), which have an important role in the management of skeletal growth. Studies have shown and proved that insulin regulates the hepatic growth hormone receptor expression to influence the serum concentrations of IGFs and IGFBPs [4,10,11].
The structural changes that characterize diabetic microangiopathy ‘abnormal growth’ and ‘impaired regeneration’ strongly suggest a role for several irrationally expressed growth factors that can possibly lead to the development of the complications. Increased concentrations have been detected and have occurred in some growth factors targeting tissues of longterm diabetic complications, enhancing the expression of the growth factors. They activate the biochemical pathways that link hyperglyceaemia to microvascular changes: vasoactive hormones; hyperglyceaemia pseudohypoxia; non-enzymatic glycation of proteins; oxidative stress, and the polyol pathway. The transforming growth factors beta (TGF-βs) are involved in both the early and late stages and is accountable for extracellular matrix (ECM) accumulation. VEGF, PDGF, and bFGF (basic fibroblast growth factor) also play crucial role in non-proliferative retinopathy as well as proliferative retinopathy. VEGF is the growth factor that is most closely correlated with neovascularization. The failure of numerous neurotrophic factors, IGF-I, and nerve growth factor (NGF) have been related to the deterioration or impaired alteration that occur in diabetic neuropathy. The liver is very vital in protecting you against diabetes. The three things that lower IGF are insulin, stress, and estrogen. Exercise, intermediate fasting, and sleep will trigger IGF [12,13].
Diabetic retinopathy can have sight-threatening complications, including preretinal neovascularization and chronic retinal oedema. Growth factors have been involved in the pathogenesis of ocular neovascularization because of the association with retinal ischaemia. The ischaemic retina secrete growth factors, stimulating residual vessels to grow rapidly. It has been found that many growth factors are manufactured during the neovascular process, resulting in limited effects of their specific inhibition. Laser photocoagulation of the retina is currently the only treatment for proliferative diabetic retinopathy because the regression of new vessels has been proven beneficial, possibly through the destruction of the ischaemic retina, which produces neovascular growth factors [13,14].
Relation between growth factors with HIV and AIDS
Human immunodeficiency virus (HIV) infection is associated with multiple defects in hematopoiesis and immune regulation. Over 36.9 million people were living with HIV/AIDS in 2017, which is the latest data available. 21.7 million people accessed antiretroval therapy (ART) in 2017 [15,16]. The deficiencies decrease the proliferation of hematopoietic progenitor cells (IGF-I, IGF-II, and IGFBP3), and a boost in the destruction of mature cells (IGFBP1 and IGFBP2). IGF-I levels are a warning sign because it increases the risk of potential adverse effects, such as malignancy or acromegalic-like symptoms. IGF-I levels can increase with other hormones such as erythropoietin for the treatment of anemia or anabolic androgens that have been used in other patients [17]. Hematopoietic growth factors are a successful approach to the management of clinical problems. Till date three major agents have been demonstrated therapeutic potential against HIV in clinical trials. In a phase I trial, granulocyte macrophage-colony stimulating factor (GM-CSF) improved pre-existing neutrophil defects and leukopenia in patients with HIV infection. In a placebocontrolled trial, erythropoietin (EPO) decreased the transfusion requirements and corrected anemia in patients who were obtaining zidovudine. In a phase I/II trial, granulocyte colony-stimulating factor (G-CSF) also corrected neutrophil defects and leukopenia in patients with AIDS excluding the alteration of HIV expression. In both treatments, G-CSF and EPO improved leukopenia and anemia, reducing the toxicity of zidovudine. There have been new solutions of hematopoietic stimulants that are being used to compress the toxicity from cytotoxic chemotherapy in the treatment of AIDSrelated malignancies [17,18].
Cytokines are involved in the pathogenesis of disease progression and in the treatment of HIV. The primary role of cytokines is to regulate blood cell production and have been examined significantly in HIV positive patients. Granulocytemacrophage colony-stimulating factor (GM-CSF), Granulocyte colony-stimulating factor (G-CSF), and IL-3 have been classified as treatments to improve the count of low blood cells that accompany AIDS. Peripheral blood cytopenia is one indication of HIV infection that has a decline in blood cell counts, resulting in immune function weakness. CD4+ lymphocytes are one of the cell types that decrease in number during HIV disease breakthrough [19].
A study performed in 2016 for reciprocal effects of “antiretroviral drugs” were used to treat HIV infection on FGF21/β-Klotho (KLB) System. Antiretroviral therapy (ART) uses HIV medicines to treat HIV (it is not a cure for the disease) and helps people live longer and healthier. It is recommended for everyone who has HIV infection to start ART as soon possible. In antiretroviral therapy (ART), results showed that HIV-infected patients showed high circulating levels of FGF21 but in contrast, β-Klotho was reduced in target tissues, making HIV a chronic medical condition. The high levels of FGF21 in HIV patients are associated with altered bone homeostasis. In present studies, the effects of FGF21 and KLB for antiretroviral treatments resulted in induction of FGF21 levels and suppression of KLB expression; reflecting the effects of gene transcription. The alterations in the FGF21/KLB system that was found in HIV patients should be improved by minimizing adverse effects of antiretroviral agents in patients that are infected with HIV [20-23].
Growth factors and Central Nervous System (CNS)
There are quite a few major classes of growth factors which act within the nervous system. Fibroblast growth factors (FGFs) are a family of growth factor signaling proteins that have multiple roles and are involved in angiogenesis, embryonic development, and signaling pathways. They also regulate developmental processes and the physiology of adults. There are members of FGF that have multiple roles and there are partially 22 members of the FGF family. 10 of them are expressed in the developing CNS, and within these 10, there are four FGF receptors (FGFR-1-4). Fibroblast growth factors -2 and -15 are commonly expressed throughout the evolving CNS. FGF-8 and FGF-17 are restricted into a specific region in the developing brain and those two are only expressed in the embryo during the early phases of neurogenesis and proliferation. FGFR- 1 is the growth factor that is expressed the most, while FGFR-2 and FGFR-3 show patterns that change expression throughout the central nervous system development. Most FGFs are fundamentally secreted using the Endoplasmic Reticulum-Golgi secretory pathway [24].
Secreted FGFs signal to target cells by binding and activating cell-surface tyrose kinase FGF receptors. FGFRs are transcribed from four different genes and consist of a single transmembrane domain, a cytoplasmic tyrosine kinase domain and an extracellular domain. There are three immunoglobin-like domains that the extracellular domain contains, named loops I, II, and III. Loops II and III attach to the bound ligand, COOH-terminal portion of loop III is the region that dictates binding in a specific matter. Another mRNA merging of the COOH-terminal portion of loop III constructs some forms of FGFRs with extremely unique ligand-binding properties. The receptor combines with a molecule to form a dimer and phosphorylates intermolecular tyrosine residues after an FGF ligand is bound, resulting in initiation trigger of FGFR signal transduction. The signaling of FGFR activates numerous of signal transduction molecules like: phospholipase C-γ pathways and Ras. FGF12 and FGF14 interact with the “mitogen-activated protein (MAP) kinase scaffold protein Islet-Brain-2 neurons” [24,25].
The binding of ligands is affected by distributing the heparin sulfate proteoglycans (HSPGs) on the cell surface and in the extracellular matrix. Extracellular FGFs bind firmly to HSPGs. HSPGs may interact with receptors on nearby cells and restrict diffusion, and favor interaction. Lastly, HSPGs promote and keep assembly in a steady state of the FGF ligand-receptor complex. Studies have shown that Xenopus embryos’ initiation of posterior neural tissue that is arbitrated by the transcription factors XBF2 and Xmeis3 depends upon the activation of the FGF signaling-dependent-Ras- MAP kinase pathways. Studies on chicken embryos that have shown that progress of posterior neural tissue is promoted by FGFs while maintaining the proliferating neural progenitors which contribute to posterior CNS development [4,24,25].
Role of growth factors in Cardiovascular Diseases
Fibroblast Growth Factors (FGFs) have recently been studied completely as some potentially new molecules in order to prevent and help treat cardiovascular disease, angiogenic actions, and attribute to metabolic effects. Members of the endocrine FGF family have been shown to increase metabolic rate, restore glucose homeostasis, and decrease adiposity. FGF serum levels have been related with well-known cardiovascular risk factors as well as with the severity and extent of coronary artery disease (CAD) and is also used to predict cardiovascular death. Clinical trials have examined FGF administration for therapeutic angiogenesis in ischaemic vascular disease. This demonstrates a possible role in order to improve ischaemic chest pain and limb function. Also, FGF21 is the most recent member of the FGF family and has recently developed as a potent metabolic regulator with multiple effects that have improved the lipoprotein profile. This may be very useful as markers of cardiovascular risk and can work as a therapeutic/ protective agent in cardiovascular disease [26].
FGF23 is a member of the endocrine FGFs and a recent large study was performed where the increased FGF23 levels were combined with cardiovascular death, including an incident heart failure in 3,627 patients with stable ischemic heart disease. There was a better response to therapy with angiotensin-converting enzyme inhibitor therapy with FGF23 levels. The link of FGF23 with direct cardio effects, including possible connotation of serum levels with cardiovascular risk factors are currently still being researched [26,27].
Growth factors and Parkinson’s Disease
Parkinson’s disease is a progressive and chronic disorder of the nervous system that affects movement of the body. It is one of the most frequent neurodegenerative disorders in humans. The cause for the disease is not clear, and no known cure has been found, but there are treatment options like medication and surgery in order to improve the symptoms. Brain-derived neurotrophic factor (BDNF) are critical modulators in maintenance and neurodevelopment of both the CNS and PNS that advocate for the survival of neuronal cells in production. These factors can likely be a potential therapeutic target in order to treat Parkinson’s disease (PD). In clinical trials, it has been reported that BDNF have been considered central in new therapeutic strategies, which can either ease or reverse the harmful symptoms in advanced Parkinson’s disease [28,29].
Growth factors and Alzheimer’s Disease
Alzheimer’s disease (AD) is the most common form of dementia that causes problems with a decline in memory, thinking and behavior. Symptoms of Alzheimer’s develop slowly and worsen over time, making it difficult to complete daily tasks. It affects more than 5.5 million Americans, about 30% of elders over 75 years old - making it the sixth leading cause of death in the U.S. There is no cure for AD, but treatments and research continue [30]. Cholinergic basil forebrain (CBF) neurons are important in pathogenesis of Alzheimer’s and are very dependent on nerve growth factor (NGF), which has been projected as a potential therapy for AD by slowing the progression of the disorder due to its effects on the CBF. Amyloid- β (Aβ) is known to impair communication between neurons, resulting in neurodegeneration. The Aβ peptides lead to deposition of senile plaques and decline the cognitive functions. Familial Alzheimer’s result from mutations in the gene for amyloid-β protein precursor or enzymes generated by Aβ [31]. It is possible for Aβ to accumulate in people with no family history of the disease, making the cause of intermittent AD less clear. Studies have shown that Aβ42 is necessary in the pathogenesis of Alzheimer’s due to its senile plaque deposits by increasing the expression of genes in neuroblastoma cells like insulin-like growth factor binding proteins 3 and 5 (IGFBP3/5). The IGFBP’s showed increase in vivo concentrations of human AD cerebrospinal fluid and are modulated by Aβ42, making it a beneficial early biomarker [32].
Growth factors and Chronic Kidney Diseases
FGF-19, FGF-21, and FGF-23 are three members of the fibroblast growth factor (FGF) family. They are endocrine factors that act in regulating several metabolic processes. They do not have FGFRs (FGF receptors), but instead have binary complexes of FGFRs and Klotho proteins. FGF-23 and FGF-21 are biomarkers that start to increase in early-stage CKD (chronic kidney disease). FGF-23 is a hormone that is mainly derived from bone, binding to α-Klotho, a transmembrane protein [22]. The physiological functions of FGF-23 are mediated from both FGF-23 and α-Klotho by forming a trimeric signaling complex, allowing to circulate and activate FGFR/ α-Klotho complexes permitting the renal tubules to increase the excretion in phosphate per neuron. In order to maintain the phosphate balance, a decrease in the nephron during the chronic kidney disease progression must be active while having an increase the FGF-23 necessary for compensation [22,33]. Nephron loss can be accelerated by inducing renal tubular damage by an increase in phosphate excretion per nephron [34]. CPPs (calciprotein particles) are a complex of calcium and phosphate nanoparticles that induce impairments such as endothelial damage and inflammatory responses [35]. CKD progression is associated by the increase of CPPs in the blood, resulting in cardiovascular disorders. Causes include CKD-MBD (CKD-mineral and bone disorder) which results in calcification of soft tissue due to damaged calcium and phosphorus metabolism. FGF-23 is elevated in CKD-MBD. FGF-21 is a liver-derived hormone that binds to βKlotho-FGFR complex that is responsible for inducing stress responses in the CNS (central nervous system), activating the SNS (sympathetic nervous system) and the hypothalamus-pituitary-adrenal axis. The most effective way for refining clinical outcomes through enrichment of CKD-MBD would include surgical interventions, which would help reduce levels of FGF-23 and CPPs [34,35].
Growth factors and Platelet-Rich Plasma (PRP)
Growth factors might have several future implications including platelet-rich plasma. Platelet-rich plasma (PRP) is a substance that promotes soft tissue healing when injected. Platelets are important reservoirs of growth factors and the role of plasma is to help blood clotting by special factors or proteins while also supporting cell growth. PRP has been produced by isolating plasma from blood and concentrating it. A few examples of PRP injections have been used for hair loss, tendon injuries, acute injuries, postsurgical repair, osteoarthritis, and more [36-39]. The following growth factors are contained in the α-granules of platelets: Platelet-Derived Growth Factor (PDGF), Transforming Growth Factor (TGF), Platelet-Derived Angiogenesis Factor (PDAF), Interleukin (IL), Vascular Endothelial Growth Factor (VEGF) (Figure 2).
Figure 2:
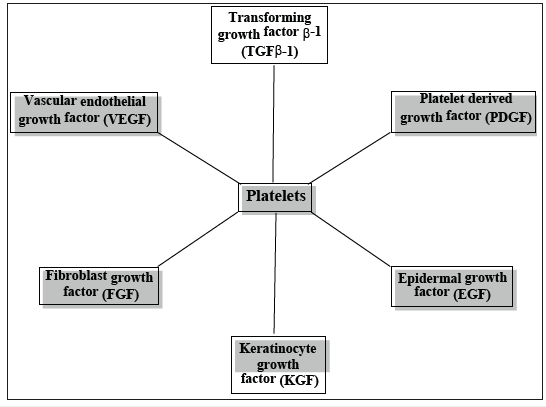
The clinical effectiveness of platelet concentrates as: Autologous Platelet-Rich Plasma (APRP) depend mainly on the concentration of growth factors, as well as the number of platelets, acting as transmitters such as tissue healing and making them responsible for proliferation, chemotaxis, tissue morphogenesis, and differentiation. Studies on the effect of PRP in hair regrowth by injecting PRP which induced proliferation of dermal papilla cells by upregulating FGF-7, extracellular signal-related kinase (ERK), β-catenin, and Akt signaling. An important factor in hair growth is anagen-associated angiogenesis due to the secretion of VEGF by the fibroblasts of dermal papilla and keratinocytes of outer root sheath. By injecting the PRP into the scalp, researchers were able to demonstrate improvement in cutaneous ischaemic conditions, epidermis thickness, and an increase of hair follicles after PRP treatment [40-42].
Conclusion
Growth factors are part and parcel in human life. They are able to initiate/propagate/maintain and terminate various cellular mechanisms. Subsequently, growth factors play important role from cell division/cell growth to cell death. Although several research projects are being conducted around the world but more research is required to understand versatile roles and functions of growth factors in human body.
Acknowledgment
The authors are grateful to the Department of Chemistry and School of Earth Environment & Marine Sciences (SEEMS) of the University of Texas Rio Grande Valley for start up funding (to DB) and for extending facilities for this study.
References
- Buch S (2014) Growth factor signaling: implications for disease & therapeutics. J Neuroimmune Pharmacol 9(2): 65-68.
- Insel PA, Amara SG, Blaschke TF, Meyer UA (2019) Introduction to the theme new therapeutic targets. Annu Rev Pharmacol Toxicol 59: 15-20.
- Stone WL, Varacallo M (2018) Physiology, growth factor. In: Stat Pearls (Eds.), Treasure Island (FL) (edn), Stat Pearls Publishing USA.
- Gunnell D, Miller LL, Rogers I, Holly JM, ALSPAC Study Team (2005) Association of insulin-like growth factor I and insulin-like growth factor-binding protein 3 with intelligence quotient among 8 to 9-yearold children in the avon longitudinal study of parents and children. Pediatrics 116(5): e681-e686.
- Raspirada A, Melillo G (2012) Role of the VEGF/VEGFR axis in cancer biology and therapy. Adv Cancer Res 114: 237-267.
- Ferrara N (2004) Vascular endothelial growth factor as a target for anticancer therapy. Oncologist 9(1): 2-10.
- Zhong J, Chen Y, Wang LJ (2016) Emerging molecular basis of hematogenous metastasis in gastric cancer. World J Gastroenterol 22(8): 2434-2440.
- Witsch E, Sela M, Yarden Y (2010) Roles for growth factors in cancer progression. Physiology (Bethesda) 25(2): 85-101.
- Zaidi S, Gandhi J, Joshi G, Smith NL, Khan SA (2019) The Anticancer potential of metformin on prostate cancer. Prostate Cancer Prostatic Dis USA.
- https://www.healthline.com/health/igf-diabetes
- Chiarelli F, Giannini C, Mohn A (2004) Growth, growth factors and diabetes. Eur J Endocrinol 151(3): 109-117.
- Dutchak PA, Katafuchi T, Bookout, AL, Choi JH, Yu RT et al. (2012) Fibroblast growth factor-21 regulates PPARγ activity and the antidiabetic actions of thiazolidinediones. Cell 148(3): 556-567.
- Bernal ME, Kulkarni RN, Scott DK, Mauvais JF, Stewart AF, et al. (2014) Human β-cell proliferation and intracellular signaling part 2: Still driving in the dark without a road map. Diabetes 63(3): 819-831.
- Gilroy CA, Roberts S, Chikoti A (2018) Fusion of fibroblast growth factor 21 to a thermally responsive biopolymer forms an injectable depot with sustained anti-diabetic action. J Control Release 277: 154-164.
- https://www.unaids.org/en/resources/fact-sheet
- https://www.hiv.gov/hiv-basics/overview/data-and-trends/globalstatistics.
- Moure R, Domingo P, Villarroya J, Gasa L, Gallego EJM, et al. (2018) Reciprocal effects of antiretroviral drugs used to treat HIV infection on the fibroblast growth factor 21/β-klotho system. Antimicrob Agents Chemother 62(6): e00029-18.
- https://aidsinfo.nih.gov/understanding-hiv-aids/fact-sheets/21/51/ hiv-treatment--the-basics.
- Miles SA (1991) The use of hematopoietic growth factors in HIV infection and AIDS-related malignancies. Cancer Invest 9(2): 229-238.
- Congote LF (2005) Monitoring insulin-like growth factors in HIV infection and AIDS. Clin Chim Acta 361(1-2): 30-53.
- Fredrick RT, Hassanein TI (2005) Role of growth factors in the treatment of patients with HIV/HCV coinfection and patients with recurrent hepatitis C following liver transplantation. J Clin Gastroenterol 39(1): 14-22.
- Quarles LD (2019) Fibroblast growth factor 23 and α-Klotho codependent and independent functions. Curr Opin Nephrol Hypertens 28(1): 16-25.
- Scadden DT, Golde DW (1996) Growth factors in the treatment of HIV disease. In: Gupta S (Eds.), Immunology of HIV Infection. Springer, Boston, USA. pp. 525-534.
- Ford PM, Abud H, Murphy M (2001) Fibroblast growth factors in the developing central nervous system. Clin Exp Pharmacol Physiol 28(7): 493-503.
- Landreth GE (1999) Growth factors are essential for nervous system development and function. In: Siegel GJ, Agranoff BW, Albers RW (Eds.), Basic Neurochemistry: Molecular, Cellular and Medical Aspects. (6th edn), Lippincott Raven, Philadelphia, USA.
- Domouzoglou EM, Naka KK, Vlahos AP, Papafaklis MI, Michalis LK, et al. (2015) Fibroblast growth factors in cardiovascular disease: The emerging role of FGF21. Am J Physiol Heart Circ Physiol 309(6): 1029- 1038.
- Wagner M, Siddiqui MA (2007) Signal transduction in early heart development (I): cardiogenic induction and heart tube formation. Exp Biol Med 232(7): 852-865.
- Jiang L, Zhang H, Wang C, Ming F, Shi X, et al. (2019) Serum level of brainderived neurotrophic factor in Parkinson’s disease: A meta-analysis. Prog Neurophychopharmacol Biol Psychiatry 88: 168-174.
- Yasuda T, Mochizuki H (2010) Use of growth factors for the treatment of Parkinson’s disease. Expert Rev Neurother 10(6): 915-924.
- Williams DM, Karlsson IK, Pedersen NL, Hagg S (2018) Circulating insulin-like growth factors and Alzheimer disease: A mendelian randomization study. Neurology 90(4): e291-e297.
- Barucker C, Sommer A, Beckman G, Eravci, M, Harmeier A, et al. (2015) Alzheimer amyloid peptide Aβ42 regulates gene expression of transcription and growth factors. J Alzheimers Dis 44(2): 613-624.
- Ning S, Jorfi M (2019) Beyond the sleep-amyloid interactions in Alzheimer’s disease pathogenesis. J Neurophysiol.
- Kuro OM (2019) Klotho and endocrine fibroblast growth factors: Markers of chronic kidney disease progression and cardiovascular complications? Nephrol Dial Transplant 34(1): 15-21.
- Vervloet MG, Sezer S, Massy ZA, Johanssan L, Cozzolino M, et al. (2017) The role of phosphate in kidney disease. Nat Rev Nephrol 13(1): 27-38.
- Akiyama K, Takaaki K, Kazuhiro S (2018) Biological and clinical effects of calciprotein particles on chronic kidney disease-mineral and bone disorder. International Journal of Endocrinology: 1-6.
- Amable PR, Carias RB, Teixeira MV, da Cruz PI, Correa ARJ, et al. (2013) Platelet-rich plasma preparation for regenerative medicine: Optimization and quantification of cytokines and growth factors. Stem Cell Res Ther 4(3): 67.
- Anitua E, Prado R, Nurden AT, Nurden P (2018) Characterization of plasma rich in growth factors (PRGF): Components and formulations. In: Anitua E, Cugat R, Sanchez M (Eds.), Platelet Rich Plasma in Orthopaedics and Sports Medicine, Springer, New York, USA. pp. 29-45.
- Gentile P, Garcovich S, Bielli A, Scioli MG, Orlandi A, et al. (2015) The effect of platelet-rich plasma in hair regrowth: A randomized placebocontrolled trial. Stem Cells Transl Med 4(11): 1317-1323.
- Lubkowska A, Dolegowska B, Banfi, G (2012) Growth factor content in PRP and their applicability in medicine. J Biol Regul Homeost Agents 26 (2 Suppl 1): 3-22.
- Fontana L, Vinciguerra M, Longo VD (2012) Growth factors nutrient signaling, and cardiovascular aging. Circ Res 110(8): 1139-1150.
- Xin J, Ding W, Hao S, Jiang L, Zhou Q, et al. (2015) Human bone marrow mesenchymal stem cell-derived hepatocytes express tissue inhibitor of metalloproteinases 4 and follistatin. Liver Int 35(10): 2301-2310.
- Kmiec, Z (2001) Cooperation of liver cells in health and disease. Adv Anat Embryol Cell Biol 161: 1-151.
© 2019 Debasish Bandyopadhyay. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






