- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Could Fast Response Curtail New Congo Ebola Virus Outbreak?
Philips Akinwole*
Biology Department, Dordt College, USA
*Corresponding author:Philips Akinwole, Biology Department, Dordt College, Sioux Center, USA
Submission: May 25, 2018 Published: June 11, 2018

ISSN 2578-0190 Volume1 Issue4
Opinion
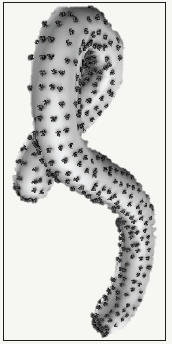
Figure 1: Ebola virus is relatively 80-nm × 800-nm enveloped (in gray) particle with a helical core (not shown) and viral proteins (shown in black) are involved in binding to and entering host cells. Ebola virus is zoonotic, which means it can be transmitted to humans from infected animals. Bushmeats and bats are known to carry Ebola virus, and the transmission of the virus from wild life populations to humans is referred to as a spillover event (i.e. spilled over into humans).
The 2013-2016 West Africa Ebola virus outbreaks was the first anywhere in the world to reach epidemic proportions - causing approximately 28,616 suspected cases and 11,310 reported deaths. The first cases of the deadly diseases were reported in Guinea in December 2013 and later spread to neighboring Liberia and Sierra Leone, with minor outbreaks in Nigeria and Mail and cases also being exported to Europe and the USA. WHO was widely criticized for its delay in taking actions to address the epidemic. Expected early alert is meant to mobilize foreign aids and actions. On August 8, 2014, WHO officially declared the outbreak a public health emergency of international concern. This declaration triggered a surge in outside help, for instance, U.S. President Barack Obama ordered up to 3,000 troops to Liberia and promised medical resources and the constructions of as many as 17 Ebola treatment centers in the region, with about 1,700 treatment beds. Britain pledged £20 million to build Ebola clinics, China sent a 59-person lab team and Cuba sent more than 400 health workers (Figure 1).
Ebola virus disease is caused by four of five viruses classified in the Filoviridae family, genus Ebolavirus with helical Five species of viruses have been established in the genus Ebolavirus: Zaire ebolavirus, Bundibugyo ebolavirus, Sudan ebolavirus, Taï Forest ebolavirus, and Reston ebolavirus. Of the four diseasecausing viruses (named for the region where each was originally identified), Zaire ebolavirus is the most dangerous and is the species responsible for the epidemic in West Africa and the latest outbreak in Congo. The name Ebolavirus is derived from the Ebola River in Zaire (now the Democratic Republic of the Congo-DRC), the location of the first outbreak in 1976.
The current, outbreak of the ebola virus in the DRC in central Africa has claimed more than two dozen lives and at least 58 others in DRC’s northwest have shown Ebola symptoms since it was identified on May 8, 2018 in a rural setting in the North-western Equateur Province. However, cases were confirmed in Mbandaka, a city of nearly 1.2 million people, this is a concerning development.
Vaccine trials in its initial stages have been launched to stop the ebola outbreak. This time around, WHO, international agencies and the DRC government sprang into action and have begun administering experimental vaccine to hundreds of people deemed to be at risk, hoping to quickly extinguish the outbreak. WHO has sent 7,540 experimental vaccines made by pharmaceutical firm Merck to DRC so far with plans to send another 8,000 doses in the next few days. The experimental vaccine was successful in a clinical trial in Guinea at the tail end of the last West Africa epidemic in 2015 and probably contributed to controlling the outbreak of Ebola virus disease in Guinea. Henao Restrepo et al. [1] in their ring vaccination cluster-randomised trial in Guinea in 2015 to assess the efficacy of a single intramuscular dose of the rVSV-ZEBOV vaccine documented that the rVSV-ZEBOV vaccine was effective in protecting against Ebola virus infection.
The ebola vaccine genetically engineered to display ebola surface proteins has not been licensed for use by any country; however, it can be given as part of a trial protocol under compassionate use regulation. As part of ebola control response, vaccinated people will be followed for 84 days to assess whether any of the vaccinated group will develop ebola disease and to evaluate possible side effects.
Since ebola has a 21-day incubation period, as soon as cases are confirmed vaccination can offer protection to the circle of people who were in contact with those infected, but also to a second ring of people who’ve had contact with the first group, and thus, potentially break the disease’s transmission chain. That’s why vaccination is really important in this Ebola response if we want to curtail the current outbreak.
WHO Deputy Director-General for Emergency Preparedness and Response Peter Salama is optimistic about the current development, “Today marks a turning point in how we deal with #Ebola - we are moving from a strategy of containment to one of offering communities protection and care” He said on Twitter.
References
© 2018 Philips Akinwole. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






