- Submissions

Full Text
Cohesive Journal of Microbiology & Infectious Disease
Malaria Disease Diagnosis-Current Status of CAD Based Approach
Sanjay Nag1 and Samir Kumar Bandyopadhyay2*
1Department of Computer Science & Engineering, JIS University, India
2Advisor to Chancellor, JIS University, India
*Corresponding author: Samir Kumar Bandyopadhyay, Advisor to Chancellor, JIS University, India
Submission: January 12, 2018; Published: January 29, 2018

ISSN: 2578-0190 Volume1 Issue2
Abstract
The CAD based approach with digital microscopy and advance image processing techniques coupled with intelligent system is the best cost effective diagnostic solution. Such software is being developed to assist pathologists and haematologist. This paper review different CAD methods.
Keywords: Rule based system; Machine learning; Morphological operation
Introduction
Malaria disease has severely affected economics of tropical underdeveloped regions and a primary cause of child mortality in such regions. This mosquito carried protozoan infectious disease can take pandemic conditions in remote rural areas within a very short time. Controlling the propagation of disease vector is often futile. The disease can only be managed with early detection, confirmation of species type, stage and density of parasite within the human blood. Manual microscopy has remained the gold standard though contemporary techniques have proved themselves to be more accurate. But considering the cost and maintenance associated with them, it is impractical to implement such technology in remote areas. Manual microscopy has a number of limitations but to overcome them technology advancements in computer science, digital electronics and microscope optics have been utilized. Digital microscopy for malaria detection is pragmatic and cost effective solution. The CAD based approach with digital microscopy and advance image processing techniques coupled with intelligent system is the best cost effective diagnostic solution. Such software is being developed to assist pathologists and haematologist. These will reduce time of observation, analysis, and inference and will provide a second unbiased, consistent opinion.
Review Works
Malaria disease is common in tropical underdeveloped countries across the globe. Most of the infections results in febrile condition but some may result in severe infection and mortality. Malaria is often called "King of Diseases" [1]. Though the disease is found to occur all-round the year it takes pandemic proportions during the rainy seasons where abundance of stagnant water gives rise to population of mosquitoes, the carrier and primary host of the disease. Though controlling the growth of mosquito is practiced everywhere but it is impossible to completely eradicate them. The disease persists and finds its way of expression and growth among human being. Better connectivity across the globe has resulted in the spread of the disease to regions where the disease was uncommon. Frequent travellers to malaria prone zones often become carriers of this disease to temperate regions of the world though strict travel advisories exist. The disease hardly results in mortality in developed countries and urban regions of underdeveloped countries but causes heavy mortality in remote rural areas where there is lack of proper medical infrastructure.
Malaria disease is known to infect humans for more than 50,000 years [2]. This disease was known as a life threatening disease and was given the name 'Malaria' in 1740. The disease possibly originated in Africa and spread to the Mediterranean region, South East Asia and India. It was very common in the marshy areas of Rome and the name originated from 'bad air' from Italian language of 'mal-aria'. The disease was also commonly known as the Roman Fever [3]. Malaria management is a challenging problem all over the globe particularly in Asian and African continents. Presently, even 110 years after the Nobel Prize of Ronald Ross for his work on malaria, people in the European region are at risk from diseases carried by vectors both within the region and when travelling abroad. While treatment of malaria itself is a challenging problem its quick detection is also a problem with no less significance [4]. Malaria remains a major global health problem with over 40% of the world's population is at risk of getting infected in more than 100 countries. Over 500 million gets affected due to malaria infections annually, causing 1-2 million deaths, majority of which are children in sub-Saharan Africa [5]. The occurrence of malaria cases is on an increasing trend due to decrease of malaria control resulting in increased transmission of the disease; the parasite has also evolved with drug resistant strains of parasites and due to increases in travel and migration [6].
The disease is known to be top on the list for causing mortality and morbidity throughout tropical and sub-tropical regions with approximately 1-2 million deaths per year [7]. The disease infected approximately 200 to 300 million people each year and the mortality rate was approximately 3 million per annum [8,9]. Statistics for the year 2010 indicated that almost half the population of the world, which was 3.3 billion people, could have fallen prey to malaria. The disease had resulted in death of 655,000 people in 2010 of which 86% being children below the age of five years [10]. Among the 62 countries which had malaria transmission in 2000 it was difficult to understand the trends of the disease in year 2000-2012. Among the rest 41 countries of 103 that accounted for 80% of the reported cases the data provided by WHO was unreliable and weak. For better understanding of the trends it is important to strengthen the information gathering and management systems to reduce the malaria burden [11].
Data show that child mortality happens every thirty seconds due to malaria so development of a malaria vaccine or the distribution, administration of appropriate and available medicine for treatment of malaria patients is essential [12]. Improper application of drugs due to incorrect diagnosis of the disease may have severe adverse effects. Similarly effective clinical trials of new anti-malaria medicine are required before application to patients as they might cause adverse therapeutic condition to patient [13]. Falciparum malaria existed from around 50,000-100,000 years but the parasite became prominent in the last 10,000 years with the advent of agriculture and increased human settlements. Studies show that the parasite initially affected gorillas [14]. Among all the species of Plasmodium known to affect humans this is the most virulent species and is majorly responsible for mortality [15]. The Malaria Vaccine Advisory Committee of WHO in 2006 proposed a "Malaria Vaccine Technology Roadmap" to develop and license a malaria vaccine with a target of 50% protective efficacy against mortality and severe infection with a lasting period of more than a year and to be developed by 2015 [14].
Proposed Method
Table 1: Morphological characteristics of Plasmodium species during different stages of its lifecycle. The images courtesy CDC, DPDx- Malaria Image Library.
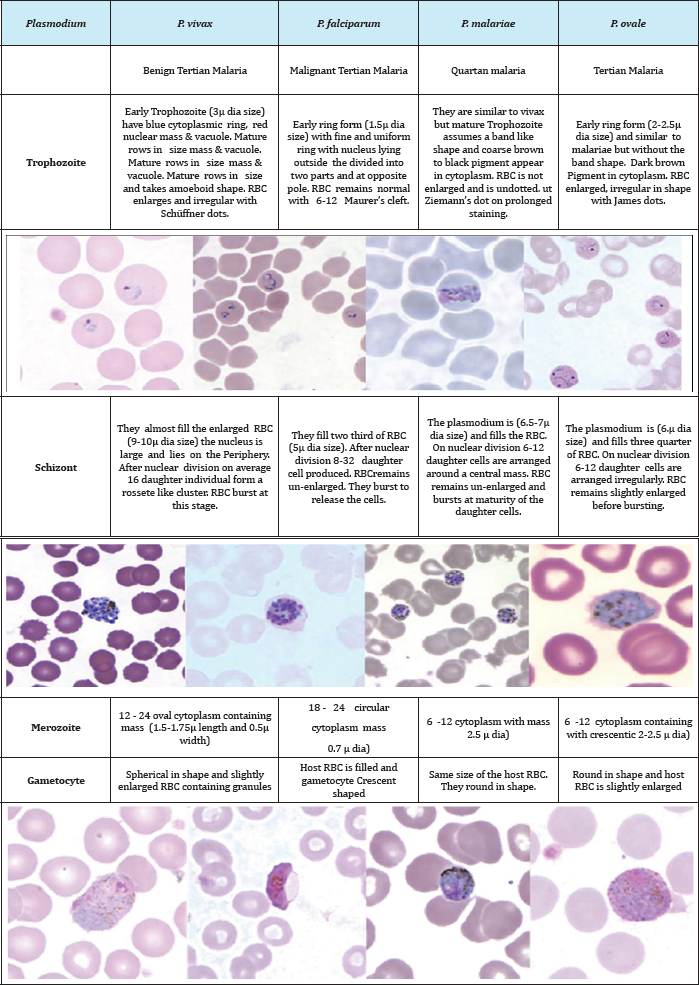
The malaria parasites belong to the Phylum Apicomplexa, Class Sporozoea, Sub-Class Coccidia, Order Eucoccida and SubOrder Haemosporina. Under this sub-order is Genus Plasmodium and this genus is characterized by the presence of two hosts in the lifecycle with Schizogony (asexual cycle) and Sporogony (sexual cycle). There are five different species affecting man. The more prevalent of them are P malariae discovered by Laveran in 1881, P vivax by Grassi and Feletti in 1890, P. falciparum by Welch in 1897 and P. ovale by Stephans in 1922. Similarly, the fifth species affecting only in South East Asia P knowlesi was discovered by Sinton and Mulliganin 1932.Tropical regions of the world between 60° N and 40° S are naturally affected by the disease. The parasite resides in two hosts namely human for asexual lifecycle and female Anopheles mosquito for sexual lifecycle. Malaria protozoa have several forms within its lifecycle. They enter the human system as Sporozoite which is a minute thread-like protozoon with tapered ends at both sides. The Sporozoites have clear cytoplasm and an elongated nucleus. They are 9-12ftm in length. Table 1 compares the differences of the different life forms of the parasite within human host [16,17].
The development of gametocyte initiates the sexual lifecycle of the parasite that takes place within the mosquito. Some infected cells instead of developing merozoites develop into gametocytes. They either form all male microgametes of size 9-10 μm or all female macrogamete of size 10-12μm. They travel to the peripheral blood vessels of the host for transmission by female anopheles mosquito vector. The different species can be identified by changes in the shape of the infected cell, by the characteristic presence of somedots like Schuffner's dots, Maurer's clefts, Ziemann's Stippling, James' dot and the morphology of the parasite during different lifecycle stages [18]. At each of the lifecycle stage, the parasite exhibit differences by its morphology, size, by the presence or absence of malarial pigment Haemozoin. Since the parasite exhibit rapid growing, often it becomes difficult to distinguish the transient stages and to classify them to a particular known stage [13].
The lifecycle of malaria parasite inhabit within two distinct hosts. Within the human host it undergoes asexual lifecycle or schizogony. Human beings are intermediary hosts while the sexual lifecycle or sporogony happens within female Anopheles mosquito, thus being the definitive host for the parasite. The development of gametes though takes place within the human host but the sexual process occurs within the mosquito.
The asexual phase within human is initiated by the injection of sporozoites through a bite by an infected mosquito. Within the human hosts it undergoes three to four distinct cycles depending on the infected species. A Pre-Erythrocytic or Primary Exo- Erythrocytic Schizogony happens within the parenchymal cells of hepatic tissues of liver. A single generation multiplication happens and merozoites are liberated called cryptozoites. The smaller micromerozoites enter the blood circulation to initiate the next cycle.
Within the blood the merozoites infects the RBC and the manifestation of the disease initiates after a definite number of days specific to each species. The ErythrocyticSchizogony cycle of development of Trophozoites, Schizonts, Merozoites and reinvasion of fresh RBC continues for several cycles at definite interval depending on the species. This is characterized by the recurrent febrile symptoms of the patient. This continues till the exhaustion of asexual life of the parasite. After achieving a certain number of asexual reproduction the merozoites develop into gametocyte or the initiation of the sexual phase or Gametogony. They generally develop within the smaller blood vessels of the internal organs like spleen and bone marrow. Mature gametes are found on the peripheral blood vessels ready for transmission by disease vector. A Latent Hepatic stage is observed in species like P. vivax and P. ovale where the merozoite enter a suspended state called Hypnozoite responsible for relapse of the disease.
Soon after a blood sucking event by female Anopheles mosquito from a malaria infected person the sexual cycle or Sporogony is initiated. A single blood meal requires more than 12 gametes/mm3 of which the female macrogametocytes should exceed the male microgametocytes. Each microgametocyte produces 4-8 threadlike microgametes whereas a macrogametocyte contains a single macrogamete. Through the process of flagellation and with the aid of chemotaxis a single microgamete comes in contact with the female macrogamete. By dissolving the wall the nuclear material of the microgamete is infused inside the macorogamete. Fertilization occurs when the two pro-nuclei fuses to form a zygote. The zygote matures to form an ookinete. This process occurs within the midgut of mosquito. The ookinete further matures and form oocyte on the stomach wall. Within the oocyte several meiotic and mitotic division results in the development of thousands of sporozoites.
Figure 1: The lifecycle of Plasmodium genus and its development in Human and Mosquito hosts.
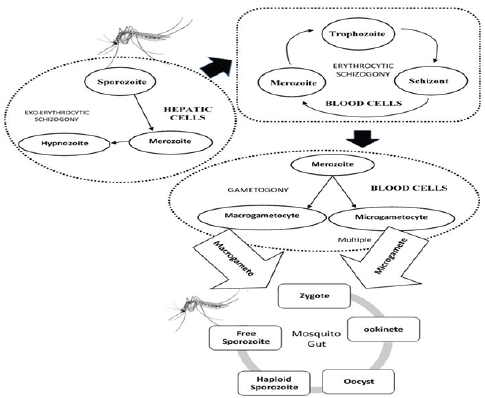
A schematic diagram is shown in Figure 1 summarizing the parasite circulation. Early and effective treatment of malaria can prevent its rapid spread among the immediate geographic location. The malaria affecting areas being mostly backward, with inadequate medical resources, thus an efficient diagnostic plan can reduce the menace and burden to society. The diagnosis of malaria identifies the presence of malaria parasite cells, antigens and antibodies within the human blood. There are different malaria lifecycle stages and five types to identify from. Moreover, diagnosis further depends upon transmission of disease, Parasitaemia, immunity, drug resistance, penetration of the parasites in the deeper tissues and other factors. Malaria in humans are diagnosed by establishing presence/absence of malaria parasite in the blood stream in adequate number, establishing the species present and the lifecycle stage of the identified parasites. Early diagnosis can prevent mortality rates. Diagnosis of malaria also depends on the availability of proper diagnostic equipment and presence of adequate trained technicians. There are areas in Africa where malaria is present among a wide population but the symptoms do not manifest and remains asymptomatic. Economical backwardness often contributes to undertrained and underpaid technicians, while underequipped medical infrastructure hinders early diagnosis of malaria.
Clinical diagnosis of Malaria
The clinical diagnosis ofmalaria is done by a medical practitioner, very often at low cost or sometimes even free of cost in government hospitals and medical centres. Malaria is often characterized by non-specific symptoms at an initial stage of infection like fever, headache, weakness, chills, dizziness, abdominal pain, diarrhea, nausea, vomiting, anorexia, and pruritus.
Such symptoms are similar to a wide range of bacterial or viral infected disease. To distinguish malaria with some other disease prevalent in the regions more specific diagnostic methods are required. It confirms that for accurate malaria diagnosis can be improved by combining clinical and parasite-based detections.
Laboratory diagnosis of Malaria
The laboratory diagnosis of malaria constitutes the most widely used mechanism for malaria detection in almost all the developing nations where malaria commonly occurs. The laboratory techniques vary in the nature of tests conducted but generally they involve collection of blood sample from the affected patient and some means using scientific equipment or chemicals to identify the presence of malaria parasite. Such laboratory tests should be done carefully and with experienced technicians. The symptoms of malaria is often non-specific in nature so wrong diagnosis in malaria prone regions resulting in over/under treatment may occur as well as false negative diagnosis in non-malaria region is a possibility. The following subsections describe some commonly used laboratory techniques for malaria diagnosis. They also describe benefits and drawback of each system and specially focus on the sensitivity, specificity, accuracy achieved by the systems. The amount of time consumed, the cost-effectiveness, and number of experts required and the fallout for lack of skilled experts are described.
Microscopic diagnosis using stained thin and thick peripheral blood smears (PBS)
Since the discovery of malaria by Laveran in 1881, use of conventional light microscopy was the primary method on blood smear slide stained with Giemsa, Wright s, or Field's stains. Staining method was discovered by Romanowsky in 1891 for enhancing the parasite for better identification. The combination of Giemsa- stained thick smear slide for initial screening and thin smear slide for species identification still remains the 'gold standard’ in laboratory diagnosis of malaria. Parasitaemia can be calculated in both types of smears.If the test reports negative detection the process is repeated every 8 hours for a couple of days to confirm non-existent of the disease. The slide preparation is vital to the method requires the involvement of skilled technicians and starts with a prick on the fingertip that was previously sterilized with ethyl alcohol. Only two drops of the oozing out blood is taken on an oil-free glass slide to prepare the smear. For thick smear a circular region is prepared with the corner of another slide. The same is allowed to air-dry and Giemsa staining of 1:20 volume is applied for 20mins. The excess stain is washed and the slide is placed vertically to dry out. The thin smear requires another slide to be held at 45 degree and swiftly moved across along the length of the slide. The slide is dried and fixed with methanol and stained with Giemsa staining of 1:20 volume for 20mins. The same is washed and kept for drying. The slides prepared are then examined under light microscope by pathologists. The WHO practical microscopy guide for malaria details the procedures to be followed by laboratory technicians.
Figure 2: Showing Peripheral smear on glass slide with and without staining taken for the purpose of microscopic studies [19].

The advantage of this system can be attributed to its simplicity, economically viability, visual differentiation between normal and parasite infected RBC, the species causing the disease and the Parasitaemia for effective disease control. The preparation of slides are however laborious, time taking and requires skill by laboratory technicians. The challenge still lies on the pathologists to identify parasite even at low level of infection. The primary disadvantage of such system is low sensitivity at detection level and species identification at low Parasitaemia. An expert technician though can detect 5 parasites/μl, on an average 50-100 parasites/μl can be detected by technicians. Absence of skilled technicians in areas where malaria is less frequently reported, availability of experts in remote areas and in cases of asymptomatic malaria or low levels of Parasitaemia contributes towards low reporting rates. Microscopy may not be available in remote areas where there is no medical infrastructure or basic healthcare and or absence of electricity. They are also useless when considered for high throughput requirements. Similarly; negative detection may arise where blood is drawn from patients on anti-malaria drugs, during the apyrexial interval of infection and first couple of days of primary infections. The presence of artefact makes the visual detection problematic. Anything other than blood component or a parasite is considered as artefact. They may include bacteria, spores, stain crystals and dirt. The presence of other blood parasites and RBC anomalies like Howell-Jolly bodies, iron deficiency, reticulocytes are considered as artefacts (Figure 2).
The rapid diagnostic test (RDT) is an important test that detects malaria quickly. A recent congress of the WHO produced a document entitled "New Perspectives in Malaria Diagnosis" (WH0/MAL/2000.1091) recommended that the results of RDT should be at least similar to the results derived from microscopy performed by a normal technician under routine field conditions. Similarly the results obtained should have sensitivity above 95% compared to microscopy findings with a Parasitaemia level of 100 parasites/l (0.002% Parasitaemia). Malaria parasites produces specific antigens proteins like histidine-rich protein II(HRP-II) or lactate dehydrogenase (LDH)that circulates in the blood stream of the infected patient The RDTs chemicals mark the presence of such antigens in the blood sample provided. They can detect a single species or multiple species. The blood for the test is obtained from a finger-prick and results are shown immediately. There are more than 200 malaria RDT kits on the market based on similar principles but they test with different chemicals and for different antigens. Currently, 86 malaria RDTs are available from 28 different manufacturers. Manufacturing of RDT kits have significantly expanded around the world. The RDT developed initially for detection of falciparum has now been adapted for vivax infection. A new RDT product launched to detect knowlesi species. Manufacturers surveyed by WHO for the World Malaria Report 2014 reported a total of 319 million RDT sales in 2013 with 59% were P. falciparum specific tests and 39% were combination tests. Rapid diagnostic tests (RDTs) represent an alternative approach to microscopy for malaria diagnosis and have shown high sensitivity and specificity. Mean operational sensitivity of RDTs based on reference microscopy was 64.8%, with the range 18.8-85.9%. Sensitivity of RDTs increased with increasing in Parasitaemia.
Table 2: Summary of Comparison between the two most popular methods.
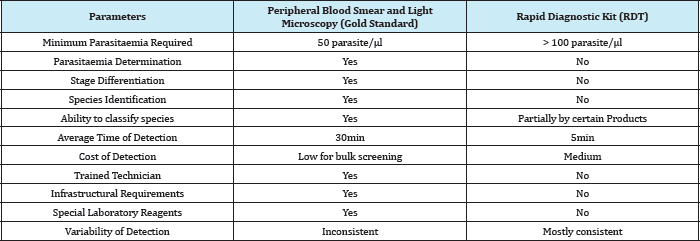
Specificity is of 87.8% regardless of poor slide quality. Malaria Antigen test using SD Bioline Malaria Antigen test that detects Plasmodium lactate dehydrogenase to diagnose vivax malaria. Data obtained from 732 patients living in areas where malaria is endemic. The platelet counts were checked. The sensitivity of the test was 96.4%, and its specificity was 98.9%. The 95.4% patients with malaria had thrombocytopenia showed a 100% positive predictive value for vivax malaria. The research citation performed a comparative analysis of SD Bioline Malaria Ag Pf. According to authors there are 86 malaria RDT products from 28 different manufacturers. They are all based on the same principle and use antibodies that detect only three groups of antigen. A popular RDT brand OptiMAL dipsticks showed a sensitivity of 96.6%, specificity of 85.4%, a positive predictive value of 92.7%, a negative predictive value of 92.6%.The use of RDT is more prevalent for regions highly prone to malaria but lacks basic medical infrastructure, electricity etc. The WHO is contemplating to develop quality control guidelines to boost the confidence of people for the use of these products. The simplicity and reliability of RDT have been enhanced so that it can be used in rural areas and regions where malaria is generally unknown or imported. Though RDT do not require specialized training or equipment the detection sensitivity is less than microscopy, nor they can distinguish among species and cannot calculate Parasitaemia. The comparison of RDT with PBS technique is summarized in Table 2.
Conclusion
Malaria disease is mostly prevalent in under-developed and economically backward regions of the tropical world. The disease can be sporadically found in other regions due to travellers visiting such malaria affected regions very often. Since the spread of the disease through mosquito bite is difficult to contain, an early diagnosis and therapeutic intervention will help to manage the disease more effectively. This research citation has described the disease and the lifecycle in details to aid better understanding of the disease. Different diagnostic tools have been extensively discussed and compared in this citation and the effectiveness of digital microscopy coupled with intelligent CAD system has been proved to be the best possible cost effective diagnostic tool. Several research works was reviewed to highlight the different image processing techniques used by fellow scientists across the world. Modern machine learning models provide better outcome and is being widely used. Methods applying such intelligent algorithms have also been discussed in the review citation. The review citation will provide adequate information for any research work in the field of CAD development for malaria detection.
References
- Makkapati VV, RaoR M (2009) Segmentation of malaria parasites in peripheral blood smear images. Proceedings of IEEE International Conference on Acoustics, Speech and Signal Processing ICASSP , pp. 1361-1364.
- Del Bimbo A, Mugnaini M, Pala P, Turco F (1998) Visual querying by color perceptive regions. Pattern Recognition 31(9): 1241-1253.
- http://rph.wa.gov.au/malaria.html
- Chakraborty K, Chattopadhyay A, Chakrabarti A, Acharya T, Dasgupta AK, et al. (2015) A Combined Algorithm for Malaria Detection from Thick Smear Blood Slides. Journal of Health & Medical Informatics 6(1): 1-6.
- Tangpukdee N, Duangdee C, Wilairatana P, Krudsood S (2009) Malaria Diagnosis: A Brief Review, Korean J Parasitol 47(2): 93-102.
- Pasvol G (2005) Management of severe malaria: interventions and controversies. Infect Dis Clin North Am 19(1): 211-240.
- Hartl D (2004) The origin of malaria: mixed messages from genetic diversity. Nat Rev Microbiol 2(1): 15-22.
- Shiff C (2002) Integrated approach for malaria control. Clin Microbiol Rev 15(2): 278-293.
- Phillips RS (2001) Current status of malaria and potential for control. Clin Microbiol Rev 14(1): 208-226.
- WHO (2011) World Malaria Report ,Geneva, Switzerland.
- Moody A (2002) Rapid diagnostic tests for Malaria Parasites Clin Microbiol Rev 15(1): 66-68.
- O'Meara WP, Hall BF, McKenzie FE (2007) Malaria vaccine efficacy: The difficulty of detecting and diagnosing malaria. Malar J 6:36.
- Arco JE, Gorriz JM, Ramirez J, Alvarez I, Puntonet CG, et al. (2015) Digital image analysis for automatic enumeration of malaria parasites using morphological operations, Expert Systems with Applications 42: 30413047.
- Kurer DA, Gejji VP (2014) Detection of Malarial Parasites in Blood Images, International Journal of Engineering Science and Innovative Technology (IJESIT) 3(3): 651-656.
- Hisaeda H, Yasutomo K, Himeno K. (2005) Malaria: immune evasion by parasites Int J Biochem Cell Biol 37(4): 700-706.
- Longo, Fauci, Kasper, Hauser, Jameson, Loscalzo, Harrison's Principal of Internal Medicine, (18th edn).
- Sachs J, Malaney O(2002) The economic and social burden of malaria. Nature 415(6872): 680-685.
- Reyburn H (2010) New who guidelines for the treatment of malaria. BMJ 340: c2637.
- http://textbookhaematology4medicalscientist.blogspot.in/2014/03/ thick-and-thin-smears-for-microscopy.html
© 2018 Sanjay Nag, et al. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






