- Submissions

Full Text
Biodiversity Online J
Management of Invasive Alien Weed (Parthenium hysterophorus L) through Allelopathic and Competitive Plants and Chemicals in Ethiopian Agriculture
Zehara MD*
Ethiopian Institute of Agricultural Research, Debre Zeit Research Center, Ethiopia
*Corresponding author:Zehara Mohammed Damtew, Ethiopian Institute of Agricultural Research, Debre Zeit Research Center, P.O. Box 32, Hora road, Debre Zeit, Ethiopia
Submission: June 21, 2023; Published: October 02, 2023

ISSN 2637-7082Volume4 Issue2
Abstract
The study conducted evaluated potential of extracts of selected plant species and chemicals on growth of P. hysterophorus. Since the impact of parthenium weed has become to the status of being an extensive economic and social problems of Ethiopian farmers. The experiment identified herbicidal potential of Aregemon mexicana leaf, stem and root extracts and 2, 4-D on seed germination and early seedling growths of parthenium at under field pot condition. Aqueous extracts of 6, 8 and 10% (w/v), obtained from dry leaves, stem root of Aregemon mexicana plants and 0.3, 0.5 and 0.7% of 2, 4-D herbicide. The overall germination, shoot and root growth means of A. mexicana extract and 2, 4-D, herbicidal treatments were significant (p<0.0001). Germination hinder by 2, 4-D and A. mexicana stem extracts, which inhibited the germination by (74.4 and 69.3%) respectively from the control (82.2%) and the lowest was A. mexicana root extract (54.8%) in invitro. In the pot experiment the overall growth parameter means of plant extract and herbicidal treatments were significantly lower than the distilled water treated plants. A. mexicana stem resulted 100% mortality at active growth stage of the parthenium plant, and 2, 4-D also showed best suppressed growth effects. A. mexicana leaf and root gave remarkable seed suppression of (93-94%) at 10% concentration. Then we conclude that species like A. mexicana and chemicals like 2, 4-D and are good candidates for future parthenium management. The Small holder farmers adopted mechanical control measures, while, it is most serious is not tackled by these farmers, integrate with other management options such as competitive plants and chemicals may appear to be satisfactory. However further research is required to progress our understanding of the interference mechanisms between parthenium and these species, on how to integrate with other management options and cost effectiveness of the methods.
Keywords:Aregemon mexicana; Herbicide; parthenium hysterophorus; Plant extracts; 2, 4-D
Introduction
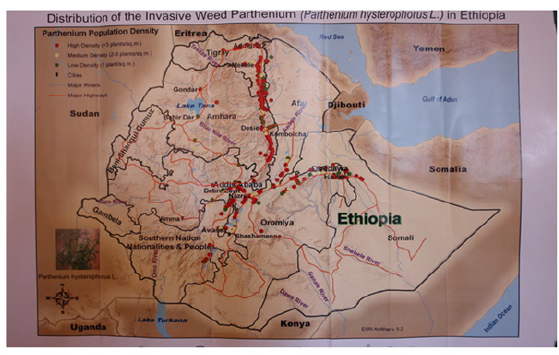
parthenium hysterophorus is an aggressive weed of family Asteraceae. It is native to the subtropics of North and South America but now has invaded Asia, Africa and Australia during the last 50 years [1]. Since its introduction in the mid-1950s, p. hysterophorus has achieved status as a major weed in India and Australia within a relatively short period [2]. parthenium has become the worst weed since its discovery in Ethiopia at Dire Dawa in Harerge province of Eastern Ethiopia in 1988 [3]. Then a second major center of infestation was subsequently found near Desse, Wello and North eastern Ethiopia. parthenium is know wide spread in Eastern Ethiopia, the central rift valley and neighboring localities of Afar Region, East Shewa, Aresi and Bale in Southern Ethiopia [4]. Similarly, from Kemisse down to Shewa Robit, dense infestation was observed along the main road, village and waterways (Figure 1). As invasive alien species parthenium can seriously affect the integrity of ecosystem by changing community structures, ecosystem function through extinction or reduction of native species, and altering ecosystem services hence productivity [5]. They may threaten native species as direct predators or competitors, as vectors of disease, or by modifying the habitat or altering native species dynamics. In the last decade, invasive alien species issues have been contentious globally due to their profound effects on agriculture, livestock and fisheries production; and also, their adverse effects on human and ecosystem health [6]. Biodiversity conservationists are currently pre-occupied with loss of native species and biological resources, and habitats because ecologically compromised habitats are easily colonized by invasive alien species, which are less preferred [7]. parthenium offers a major challenge to all attempts of control because of its high regeneration capacity, production of huge amount of seeds, high seed germinability and extreme adaptability to a wide range of ecosystems [8]. Several methods are being recommended in suppressing the growth of parthenium but none of them appeared to be satisfactory, as each method tried during the period from mid-sixties to eights suffered with one or the other limitations such as inefficiency, high cost, impracticability, polluting the environment, temporary relief. The various approaches employed so far include manual, chemical, biological and integrated methods [9]. Recently some studies carried out have shown very encouraging results regarding the use of allelopathic plants in parthenium management. [10,11] showed that the allelopathic grasses Imperata cylindrica, Dicanthium annulatum, Cenchrus pennisetiformis and Sorghum halepense. There are hundreds of secondary metabolites in the plant kingdom and many are known to be phytotoxic [12]. Allelopathic effects of these compounds are often observed to occur early in the life cycle, causing inhibition of seed germination and/or seedling growth. The compounds exhibit a wide range of mechanisms of action, on DNA, photosynthetic and mitochondrial function, and phytohormone activity, ion uptake and water balance. In the course of an extensive survey carried out to assess the distribution of parthenium in India in the years 1987-1990 [13], it was observed that parthenium does not grow in proximity to particular plant species. This suggests a natural antagonism of these species towards parthenium [14]. Well known herbicides such 2, 4-D sodium salt, arsenate compounds, paraquat, bromacil, glyphosate and sodium chloride are reported to kill the standing parthenium populations [9].
Figure 1:Map for distribution of parthenium in Ethiopia. Source: Map prepared by collaboration of USAID, Virginia State University, Excellence Research and development, EIAR, Haramaya and Mekelle University.
According to [15]. Halosulfuron, MSMA, bromoxynil, 2, 4-D, and flumioxazin controlled 58 to 90% rosette R. parthenium at 3 weeks after treatment. R. parthenium control with all other post herbicides was less than 38%. At bolted-stage, glyphosate, glufosinate and trifloxysulfuron controlled 86 to 95% R. parthenium and control was 61 to 70% with chlorimuron, halosulfuron and 2, 4-D. In Ethiopia, spraying 2kg/ha of 2 4-D sodium salt or 2L/ha MCPPA in 400L of water were found effective to control parthenium at seedling stage [16]. However, the small holder farmers in the study area adopted mechanical control measures like slashing in farm lands; the problem in wastelands, plantation, irrigation and drainage ditches, fence lines, poorly managed crop fields, roadside, park and grass land where it is most serious is not tackled by these farmers. Management efforts including competitive plants will at best delay widespread processes. Hence, study conducted on suppression of this invasive weed is of paramount importance for the resource poor farmer mainly and different stakeholder in terms of giving feasible solution to the existing problem. In addition, it could help to fill the gap of information for those concerned, to study controlling methods in other parts of the country.
Materials and Methods
Site description
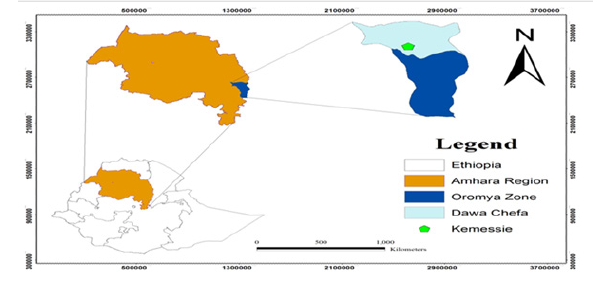
The field study was conducted in Amhara Region, Oromia Zone, Dawa Chefa Woreda in central part of Kemisse town, which is the zonal capital of Oromia zone. Geographically it is located 10043’32” N latitude and 39052’52”E longitude. The altitude of the area ranges from 600 to 3200m above sea level and the mean annual rainfall ranges from 600-1100mm. The texture of soil of area at 0-50cm depth is loamy sand near the hill and loam at the alluvial deposit (downstream of the catchments) and sandy loam at 50-100cm depth on both locations. The population of the Woreda is estimate a total of 147000 residents [17]. The invitro test was conducted in Meklle University (Figure 2).
Figure 2:Map of Ethiopia and the study site Kemisse.
Experimental procedure
For A. Mexican, leaf, stem and root parts matured plants were collected from heavily infested range land by Aregemon mexicana around Borkena valley near to Kemisse and the parts were carefully separated. After thorough washing with water, it was kept to dry in light shade. Then prior to extraction the plant parts were kept to dry well to its appropriate moisture level at the laboratory of Leather and Leather product Institute of Maazama oils in Addis Ababa. In most cases the moisture content of the material was below 12% (personal communication with chemists). The samples were chopped into pieces using grinder for extraction. Each sample (10g) were weighted using aluminum tray and transferred to a juice maker, distilled with 100ml per sample and run to extract for 10 minutes by using blender mode [18] to obtain a10% (w/v) aqueous extract, 10g crushed dry leaf, stem and root material A. Mexican was soaked in 100ml). The whole materials were then transferred into flask and placed on a shaker for 10 hours. The extracts were allowed to settle for 1hrs before filtration. Then (95%) ethanol is added to the filtrate to avoid the growth of fungus. Further final dilutions of 8%, and 6% [18], were prepared by adding appropriate quantity of distilled water to the 10% stock solution, then the dilutions were mixed by shaking for certain minuets and stored at 4oc. For 2 4-D herbicide dose level 0.3, 0.5% and 0.7% were taken considering literature on herbicides such as glyphosate (0.75- 1.00%), metribuzin (0.3-0.5%), 2 4 D (0.5%) have given satisfactory control of the weed under diverse agro climatic conditions [19].
Treatment application
The top of paper method was used for germination test of parthenium seed. Petridish (9x18cm size) were washed with 70% ethanol and cleaned three times with distilled water and incubated until it dries for Invitro test. The Petridish were arranged in completely randomized designed design with three replications. The moistened capacity of filter paper was checked by distilled water, the filter paper lined in each sterilized Petridish moistened with 1.2ml of different extracts and thirty seeds of parthenium in each Petridish were arranged on the top of moistened paper and placed in 23 ℃ temperature. The control treated similarly with distilled water and the plates were regularly checked for moisture. The solutions of different extracts and chemicals were applied to each plate ever two days. parthenium seeds were seeded on 0.125m2 large plastic bucket seed bed for transplanting purpose. Thus, another pots (27x25cm size) were filled with 2kg of sandy loam, 3kg of decomposed forest soil and 3kg of black top soil from Dewa Chefa nursery site under natural condition. When the seedling attended three to four true leaves on, two parthenium seedlings in each pot were transplanted to minimize the risk of germination failure on the above pots. The seedlings were watered every two days interval starting from sowing up to the stage of maturity. Finally, when the seedling was reached at actively growth stage. By taking a single plant checked the amount of solutions that well moistened whole area of the leaf using distilled water. Then one plant was well moistened by 40ml treatment solution, so for two parthenium seedling in a single pot 80ml extract were sprayed. Similarly, the second round spray were carried out after three weeks of the first spray at flowered plants (Figure 3).
Figure 3:First round spray at actively growth stage.

Data collection
The number of seeds exhibiting radicle and plumule emergence were recorded at 3 days interval during the germination test period of 15 days. In addition, shoot and root length were measured by taking 10 randomly selected plants for each Petridish for Invitro test. For natural field pot experiment plant height were measured from pot ground level to the top of the shoot. The numbers of dead plant were recorded for two months after spray to know the mortality date of the plant after spray. In addition, the spikelet that drop every day at maturity were recorded for the last 20 days of maturity since the plant has characteristics of some spikelet’s drop before the whole plant fully matured and finally when the whole plant fully matured the whole spikelet was counted. The biomass was taken by measuring above ground whole weight of the plant. At end the seed yield were measured by golden Bean balance.
Statistical analysis
The collected row data’s normality was checked, and then transformation performed. After data transformation for all growth parameters of invitro and field pot data the analysis of variance, coefficient of variation and comparisons of treatments means were done by using SAS version 9 computer software, at probability significance level of α=0.05 (SAS Institute, 202).
Result and Discussion
Invitro test
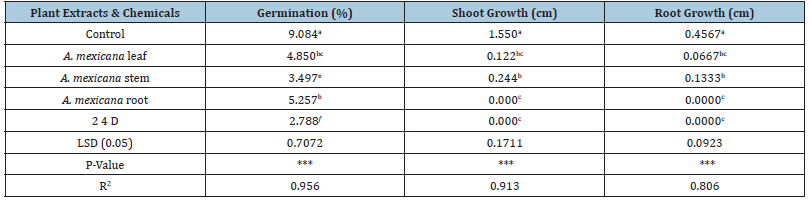
Analysis of invitro test data showed that all treatments (plant extract and herbicides) had significant (P<0.0001) inhibition effect on germination, shoot and root growth of parthenium (Table 1). The overall seed germination means of plant extract and herbicidal treatments were significantly lower than the water treated (controls), the highest germination suppression was and 2, 4-D, and A. mexicana stem extracts; and the lowest was A. mexicana root extract. The findings indicated that the effective treatment in suppressing germination was 2, 4-D herbicide, where, germination was reduced by (74.5%) compared to control (82.2%). Moreover A. mexicana stem reduced germination by (73%) compared to control (Table 1). The effect may probably due potential disrupting of chemicals on the plant’s ability to germination. The above results indicated that allelopathic effect of various plant extracts from different parts and chemicals appeared to be different depending on the plant species and chemicals. Such difference may be related to specific allelopathic compounds being produced in each plant parts. [20] studied the herbicidal effect of volatile oils from leaves of E. citriodora against the noxious weed P. hysterophorus and found that a concentration of 5.0nL ml-1 Eucalyptus oil completely inhibited the germination. [21] have reported significant suppression of Physalis angulata L., a problem weed in maize, cotton and soybean fields in Turkey, by aqueous extracts. Shoot and root growth was highly suppressed by all applied plant extract and chemical. The damage was most pronounced by A. mexicana root and 2 4-D, each which totally suppressed shoot and root growth (Table 1). The reductions in seedlings root and shoot length may be attributed to the reduced rate of cell division and cell elongation due to the presence of allelochemicals in the aqueous extracts which ultimately reduced elongation. Generally, the findings revealed that all tested herbicide inhibit germination and early growth, the effect was most pronounced by 2, 4-D chemicals, followed by A. mexicana plant extracts compared to control (Distilled water) treated seeds.
Table 1:Effect of plant extracts and chemicals on germination shoot and root growth.
Pot experiment under natural condition
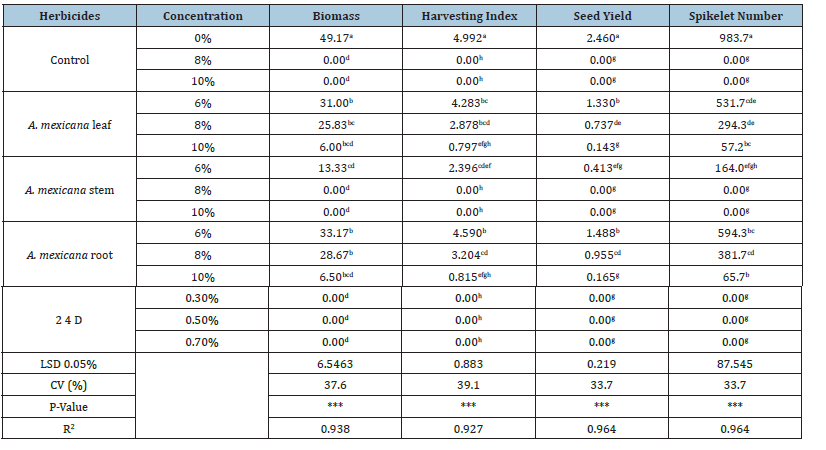
Analysis of the pot experiment data revealed that the tested A. mexicana plant extracts and chemicals had significantly (P<0.0001) reduced biomass, harvesting index seed yield, number of spikelet’s per plant and increased mortality percentage of parthenium plant (Table 2). Moreover, effect of different concentration was significantly varied in their effects on the growth parameters of parthenium. The overall growth parameter means of plant extract and herbicidal treatments were lower than the water treated (controls) plants. 2, 4-D at all concentration level and A. mexicana stem extracts concentration resulted 100% mortality at active growth stage of the parthenium plant. The six above treatments had the maximum potential to inhibit the different growth parameters of parthenium weed.The highest biomass suppression was A. mexicana stem and modest suppression was A. mexicana root extract. The findings revealed that at 10% concentration A. mexicana leaf and root extracts remarkably suppressed biomass of parthenium by (87.8 and 86.7%) from the control (49.17gm per plant); followed by A. mexicana stem at 6% was also providing effective reduction on biomass by 73%) compared from the control (Table 2). According to reported that aqueous extracts of A. indica leaf of low concentrations were least toxic exhibiting not strong impact on biomass. At 10% concentration A. mexicana leaf and root extracts remarkably suppressed parthenium seed production by (94.2 and 93.2%) respectively from the control (2.46gm per plant) (Table 2). The concomitant decrease in seed yield of the plant may be explained by the fact that as less shoot and root fresh yield was produced by the plant, less assimilates were partitioned to the shoot portion and resulted in less photosynthesis, thereby decrease seed yield. This may also be associated to the lower number of branches per plant, number of capsules per plant, and number of seeds weight per capsule, which thus contribute to reduced seed yield. The mode of action might be by inhibiting respiration energy then Chlorosis and necrosis cause loss of chlorophyll from leaves. Cause photo bleaching of chloroplasts and allelochemicals. Inhibition of photosynthetic process depletes food reserves, and then proteins and other compounds can serve as respiratory substrates [9]. Among tested treatment A. mexicana root and leaf extract at 10% reduced by (4.17 and 4.2) the harvesting index respectively compared to control 4.99 (Table 2). Followed by, A. mexicana stem at 6% reduced the harvesting index by (2.6) compared to control. This could be most likely due to assimilation and utilization of growth factors after extract application might have disfavor the movement of nutrients in the plant and stressed growth of productive tillers this result decrease in the harvesting index. At 10% A. mexicana leaf and A. mexicana root, and at 8% A. mexicana stem, reduced the number of spikelet’s per plant by (927, 916, 717) respectively compared to control 983. This could be due to the development of short stature, lower number of leaves as well as lower number of branches resulted from spray of plant extract might have an effect on flower development and finally on the number of spikelet’s per plant.
Table 2:
The survival existence means of plant extracts after spray were significantly lower than the water treated (controls). In case of A. mexicana stem treated plants, the plant died within 5 days and 6 days respectively (Table 2). Effects of herbicides were monitored till all the plants in herbicidal treatment are died. Since the plants did not regenerate after death that also increases the importance of these herbicides for their selection against parthenium. This might be resulted due to more and vigorous allelopathic potential of plant that disfavor regeneration of parthenium weed (Figure 4). The present study results showed consistence effect at invitro and pot experiments. Interestingly both the invitro and pot result realizes the observation of poor parthenium density near exotic tree plantation such as A. mexicana and waste land weed like A. mexicana. So allelochemicals release from living dead plant materials of thesis tree and weeds accumulate in the soil beneath adversely affect the germination and growth of parthenium. The present study also revealed that the possibility of using the allelochemicals directly or as a structural lead for the discovery and development of environmentally friendly bioherbicide and chemicals to control one of noxious weeds of P. hysterophorus in the country.
Figure 4:parthenium plants sprayed with A. mexicana stem.

Conclusion
The study identified that germination, shoot and root growth of plant extract and chemical treatments were significantly lower than the control (distilled water). The invitro findings revealed that the tested plant extract and chemicals suppressed the germination, shoot and root growth, the effect was most pronounced by 2, 4–D chemicals, followed by A. mexicana plant extracts compared to control (Distilled water) treated seeds. Further the pot study revealed that 2, 4- D, and extracts from A. mexicana stem at 8 and 10% concentrations resulted 100% mortality at actively growth stage of the parthenium plant. In addition, the plant extracts indicated the highest suppressions on different growth parameters and seed production of parthenium plant. From the results of the present investigation we conclude; that species like A. mexicana some chemicals like 2 4-D, are good candidates for future P. hysterophorus management.
Acknowledgement
The study was financially supported by the Rural Capacity Building Project (RCBP), Ethiopia. The helps of Leather and Leather product Institute of Maazama oils Addis Ababa, Ethiopia during the extracts preparation and for providing us extract oils is thankfully acknowledged.
References
- Javaid A, Shafique S, Shafique S (2007) Causes of rapid spread of parthenium hysterophorus L. and possible control measures in Pakistan-A review. Pak J Bot 39(7): 2611-2618.
- Dhileepan K, Senaratne KW (2009) How widespread is parthenium hysterophorus and its biological control agent Zygogramma bicolorata in south Asia. European Weed Research Society Weed Research 49(6): 557-562.
- Tamado T (2001) Biology and management of hysterophorus L. in Ethiopia. Sweden.
- Rezene F, Meckasha C, Mengistu H (2005) Spread and ecological consequences of Parthenium hysterophorus, in Ethiopia. Arem 6: 11-21.
- Taye T (2007) The prospects of biological control of weeds in Ethiopia. Eth J of Weed Mgt 1(1): 63-78.
- Kuma E (2008) Perceived socio-economic impact of parthenium weed and its effect on crop production Ethiopia. Ethiopia.
- Odeny D, Lusweti A (2009) UVIMA Baseline Review Consultancy report: Invasive and alien species in Kenya.
- APFISN (2007) Carrot weed: Parthenium hysterophorus.
- Wahab S (2005) Management of parthenium through an integrated approach initiatives, achievements and research opportunities in India. Pp: 55-59.
- Anjum T, Bajwa R, Javaid A (2005) Effect of Imperata cylindrica on distribution, germination and seedling growth of hysterophorus L. Int J Biol Biotech 2(2): 459-46.
- Javaid A, Anjum T (2005) Parthenium hysterophorus L-A noxious alien weed. Int J Biol Biotech 2(2): 459-463.
- Einhelling FA (2002) The physiology of allelochemicals action.
- Aneja AK (1991) Deadly weed Parthenium hysterophorus and its control. Egyptian Journal of Biology (12): 57-64.
- Knox J, Jaggi D, Paul MS (2010) Evaluation of allelopathic potential of selected plant species on Parthenium hysterophorus L. Egyptian Journal of Biology 12: 57-64.
- Reddy KN, Bryson CT, Burke CI (2007) Ragweed parthenium (Parthenium hysterophorus) control with preemergence and post emergence herbicides. Weed Technology (21): 982-986.
- Taye T (2002) Investigation of pathogens for biological control of Parthenium hysterophorus L. in Ethiopia. Germany.
- BOA (2000) Report on area, agroclimate and major crop production forecast for 2001/2002 cropping season on private peasant holdings Amhara Region Kemisse, Ethiopia.
- Shafique S, Bajwa R, Javaid A, Shafique S (2005) Biological control of parthenium IV: Suppressive ability of aqueous leaf extracts of some allelopathic trees against germination and early seedling growth of hysterophorus L. Pak J Weed Sci Res 11(1-2): 75-79.
- Yaduraju NT, Sushilkumar MBB, Babu P, Gogoi AK (2005) Parthenium hysterophorus distribution, problems and management strategy in India. International Conference on Parthenium Management Bangalore, pp: 6-10.
- Singh HP, Batish DR, Setia N, Kohli RK (2005) Herbicidal activity of volatile oil from Eucalyptus citrodora against hysterophorus. Annals of Applied Biology146: 89-94.
- Uremis I, Arslan M, Uludag A (2005) Allelopathic effects of some Brassica species on germination growth of cut leaf ground cherry (Physlis angulata L). Journal of Biological Sciences 5(5): 661-665.
© 2023 Zehara MD. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






