- Submissions

Full Text
Biodiversity Online J
Ochna Pulchra as an Alternative Raw Material for Biodiesel Production
Charles M1*, Chiyapo G1 and Kinya A2
1Department of Agricultural Research, Botswana, Gaborone
2University of Tottori, Japan
*Corresponding author:Charles Mazereku, Department of Agricultural Research, Botswana, Gaborone
Submission: May 21, 2023; Published: May 29, 2023

ISSN 2637-7082Volume3 Issue4
Abstract
Biodiesel as a plant-derived fuel can be sustainably produced domestically and globally. Botswana has a wide range of oil bearing plants that can be found in the wild. The plants cover all districts of the country. Ochna pulchra is one of the available oil bearing plant and well distributed in the entire country. A large population of mature plant was located in Kweneng District. The local people were engaged to collect the seeds for oil extraction. Ochna spp. extracted oil was a black, smelly oil and biodiesel is with light brown with out discernible odour. The seeds produce about 45% with a high content of free fatty acids (10%). Production of biodiesel from this plant oil involves two-step processes (esterification and transesterification). It was found that, the physico-chemical properties of produced biodiesel were within the established international standards; density was 887kg/m3 versus 860-900kg/m3 ASTMD 1298. The flash point was 153°C versus 120°C minimum ASTMD93 and the energy content was 38.64kJ while the established standard is 42kJ. Ochna pulchra has a great potential to serve as feedstock for biodiesel production because its diesel is desirable or comparable to fossil diesel.
Introduction
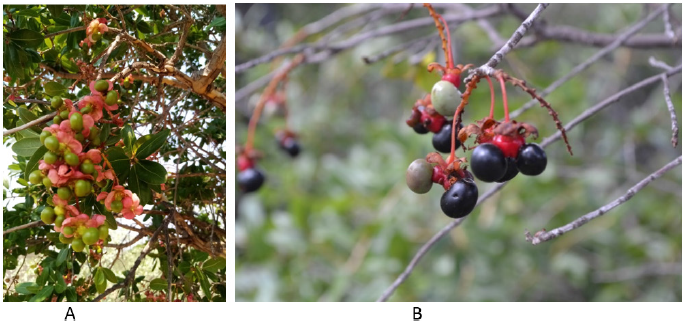
Biodiesel is a liquid biofuel obtained from the chemical processing of vegetable oil, used as a cooking oils, animal fats and fats [1]. Many plant species such as Jatropha carcus, castor bean, sunflower, soybean, rapeseed, etc. have been successfully tested and used as a raw material for biodiesel production [2]. However, production of biodiesel from the above materials requires a large volumes of raw-materials that also requires a large piece of land being cultivated which ultimately increase production costs [3]. Besides that, there are other low cost, readily available wild oil bearing plants such as Trichilia emetica, Pappea capensis, Croton megalobotrys and Ochna pulchra, that have never been explored for biodiesel production. Among the listed indigenous plants Ochna pulchra (Figure 1) is well distributed all over the country [4] and it is found in, Namibia, South Africa, Zambia, Mozambique and Zimbabwe. It has different native names across countries, but in Botswana, it is commonly known as Monyelenyele and Kyara [4,5]. It is a single smooth, flaking trunk, evergreen tree growing to 10m tall. The tree has pale, greenish-yellow flowers and bears black, kidney-shaped berries. The berries give off an unpleasant-smelling oil that can be obtained by boiling the seeds. The oil from the Ochna Pulchra seeds used by the Basarwa and Hambokushu tribe as cooking oil [5]. It blossoms and bears fruit around November - December and ripen around January –February. In Botswana a large population of the tree was located in Kweneng district (Mmatshwabisi, Monwane), Southern (Jwaneng Sese), and sparse population in other districts of Botswana. Therefore, the plant can be explored to supplement the available feedstock for biodiesel.
Figure 1:Ochana pulchra tree a) Fruits are still green (October). b) Fruits are black, ripe (January).
Material and Methods
Site description and seed collection
The seeds collection site was done between Molepolole and and Letlhakeng village. The area of collection is bush veld dominated by Ochna pulchra and the Terminalia spp, Grewia spp and Eragrostic pallens grass. The soil is Kalahari sandy soil. A settlement between Molepolole and Letlhakeng villages has been identified for gathering berries. Only a few residents of the settlement were approached and commissioned with the collection. The collection was conducted from January 18 to March 17, 2021. A total of 100kg was collected. The seeds were sun dried for two weeks (Figure 2) and then oven dried at 50°C for three hours before the oil was extracted from the berries.
Figure 2:Sun dried ochna berries.

Chemical quantification of oil content in Ochna pulchra seeds
An Ankon XT15 chemical extraction machine (petroleum ether as a solvent) was used for chemical quantification of oil yield in Ochna berries. Dried berries were ground into a powder form (2mm). The ground powder of about 1 to 2g weighed into a preweighed filter bags. The sample first was recorded as Weight 1 (W1). Three samples were weighed and then placed in a carousel that was loaded into the extraction machine. The machine was run for 60 minutes and the samples were removed and dried in a desiccator for 30 minutes. The samples were weighed to record the second weight (W2). The oil yield was calculated by a formula W1- W2/W1*100.
Oil extraction from Ochna berries
A cold press machine model BGC T15 was used. The berries were thoroughly checked for small pebbles prior to extraction. The machine gets to malfunction in the presence of pebbles. At least the machine hopper is able to accommodate 5kg of the berries and the machine takes about 45 minutes to complete the batch. The machine was able to release 2 litres of black coloured, unpleasant smelly oil from 5kg of seeds. A total of 30 litres were produced from this extraction.
Convention of the raw oil to biodiesel
About 2 litres of oil was boiled for 10 minutes at 110 oC to get rid of moisture, moisture tend to interfere with the process of convention of oil to biodiesel. The oil was then left to cool to a room temperature.
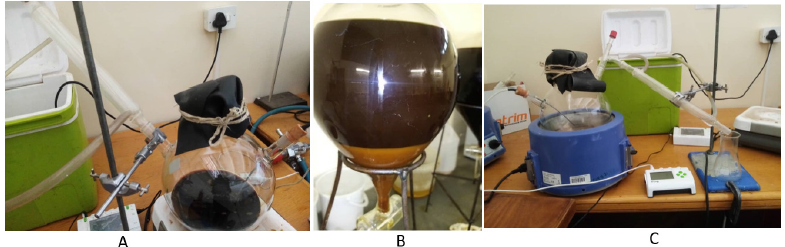
Transesterification of ochna oil to biodiesel:Acid value of the raw oil was checked which was found to be at 15%, at that level the acid value is said to be too high. A two-step method is to be followed (Table 1), the first step will be to reduce the acid value to about 1% or below then the second stage will be to perform transesterification [6]. The first step is called esterification where a 20% (w/w) methanol is mixed with the raw oil in the presence of 3%(w/w) sulphuric acid. A crude oil weight of 1701.6g, methanol 20% (w/w) of 340.32g and H2SO4 3% (w/w) of 51.048g were placed in a reaction chamber. The reaction chamber was placed on a hot plate with a magnetic stirrer. The temperature was maintained at 40°C for 1 hour. The solution was then allowed to cool to room temperature and then placed in a separator overnight (Figure 3). The bottom segment of the solution was discarded and the top layer was taken further to the next stage. Before proceeding to the next step, free fat acid was checked to if it was reduced to 1% (Table 2). The transesterification process involved these materials; 1622.8g of the esterified crude, methanol 324.56g (20%) (w/w) and potassium hydroxide from 16.228g (2%) [6,7]. The oil was placed in a reaction chamber (Figure 4A) and the temperature was maintained at 40 °C while potassium was dissolved in methanol to produce methoxide. The methoxide was added to the oil and the temperature was raised to 60 °C and held for 1 hour. The solution was then allowed to cool to room temperature and later poured into a separator (Figure 4B) to stay overnight. The separator separated the solution in two layers, with the top layer being the biodiesel and the bottom layer being glycerine and other contaminants. The bottom layer was removed and set aside while the top layer was removed for further processing. The biodiesel was placed in a reaction chamber (Figure 4A) and the temperature maintained at 70 °C to recover methanol, the process being so until no methanol dripped. The remaining biodiesel was weighed and it weighed 1495.047g which translate to 92% convention rate.
Table 1: Results for oil content in Ochna puchra seed.
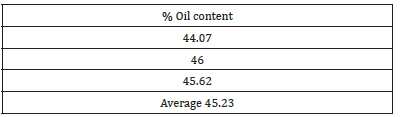
Figure 3:Esterified ochna oil in a separator.

Table 2: Free fatty acids results.
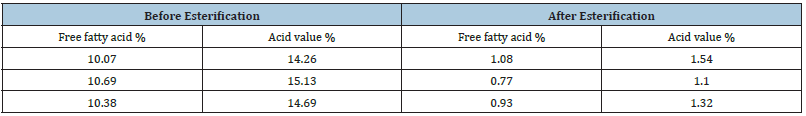
Figure 4:Transesterification process
a) Reaction chamber
b) Separator
c) Removal of methanol from crude biodiesel
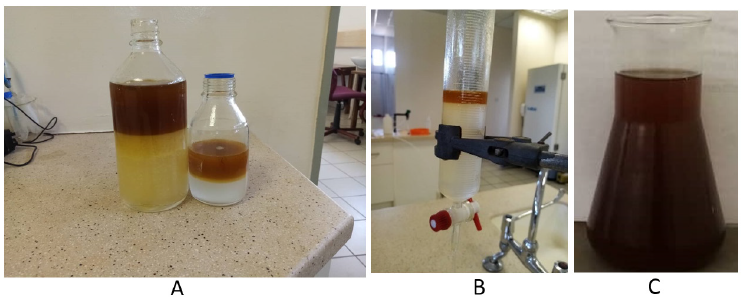
Washing of the biodiesel:A warm distilled water was mixed with the diesel to wash off un reacted material and other impurities. The separator was used to separated water from diesel (Figure 5A). The washing continued until the water was clear (Figure 5B). The biodiesel was air dried to get rid of leftover moisture.
Figure 5:Washing of crude biodiesel a) warm water is mixed with crude biodiesel and shake b) the mixture poured in a separator to separate water (lower layer from biodiesel upper layer c) a dark brown biodiesel is obtained
Analysis of the biodiesel
Some analysis was performed to the biodiesel just to qualify it to the set international standards of biodiesel (ASTM AND EN) [8]. The parameters analysed were density, energy content and flash point. Density was measured using density meter, KEM Kyoto electronics density machine, energy content was measured using calorimeter model 3K-1 while flash point was measure using flash point machine model AMP- 8fc.
The Results and Discussions
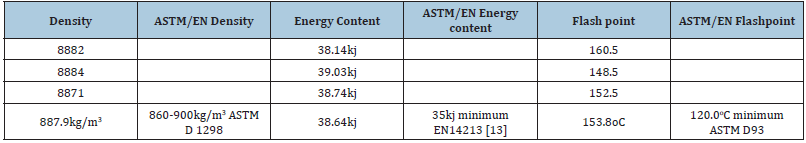
Ochna pulchra berries have a high crude oil content (45%) as reported in Table 1. Compared to other oil crops, their yield is still below that of palm, olive, but on par or better than sunflower and soybean [9]. Ochna pulchra Crude oil has a free fatty acid of 10% (Table 2) and Jatropha curcas has 21% [10], while used cooking oil accounts for 16.4% [11]. [12] Vegetable oils or waste oils containing more than 1% free fatty acids have to go through two steps in biodiesel production, the first step is to reduce the acidity with a strong acid as a catalyst and the second step is to use potassium as a catalyst [10]. The physicochemical properties of Ochna pulchra derived biodiesel with density: 887.9kg/m3, energy: 38.64kJ and flash point: 153.8 C (Table 3). The physical chemicals of biodiesel fall under the internationally established standards for biodiesel [13]. Figure 6 shows that ochna oil has a higher (44%) content of palmitic acid followed by oleic acid (32%). The fatty acid composition is very different from that of Jatropha carcus, which has a higher content of oleic acid (44%), followed by linoleic acid with 34% [14]. The fatty acid results also compared well with Palm oil, which consists mainly of palmitic and oleic acids, 42% and 41% respectively [15]. The table shows the physicochemical properties of Ochna derived biodiesel conform to the international biodiesel standards (ASTM/EN).
Figure 6:Fatty composition of Ochna pulchra oil.
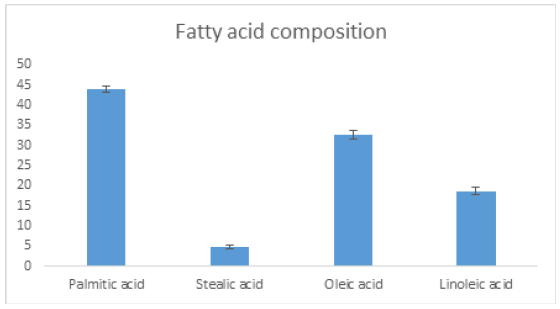
Table 3:Results for physicochemical properties of biodiesel.
Conclusion
Biodiesel from Ochna pulchra berries is suitable and it meets the most important international standards for biodiesel. The Ochna pulchra tree is readily available in most parts of Botswana, making it a good candidate as a feedstock for biodiesel production. Although the oil is edible in some parts of the country, it is used sparingly and other edible oils can be used, then ochna oil can be fully used for biodiesel production. The Ochna pulchra can further be improved agronomical so that it can yield more berries and oil yield.
References
- Tamalampudi S, Fukuda H (2011) Comprehensive Biotechnology. Second Edition 3: 63-70.
- Demirbas A, Bafail A, Ahmad W, Sheikh M (2016) Biodiesel production from non-edible plant oils. Energy Exploration & Exploitation 34(2): 290-318.
- Mizik T, Gyarmati G (2021) Economic and sustainability of biodiesel production-A systematic literature review. Clean Technologies 3: 19-36.
- Setshogo MP, Venter F (2003) Trees of Botswana.
- Letloa LLHRC (2007) The Khwe of the Okavango panhandle: The use of veld plantas of food and medicine.
- Meka PK, Tripathi V, Singh RP (2007) Synthesis of biodiesel fuel from safflower oil using various reaction parameters. J Oleo Sci 56(1): 9-12.
- Gandure J, Ketlogetswe C, Temu A (2014) Fuel properties of biodiesel produced from selected plant kernel oils indigenous to Botswan: A comparative analysis. Renewable Energy 68: 414-420.
- Prankl H, Körbitz W, Mittelbach M, Wörgetter M (2004) Review on biodiesel standardization world-wide. IEA Bioenergy Task 39: 38-46.
- Huang D, Zhou H, Lin L (2012) Biodiesel: An alternative to conventional fuel. Energy Procedia 16(C): 1874-1885.
- Win SS, Trabold TA (2018a) Sustainable food waste to energy systems. Sustainable Food Waste-to-Energy Systems 89-109.
- Banani R, Youssef S, Bezzarga M, Abderrabba M (2015) Waste frying oil with high levels of free fatty acids as one of the prominent sources of biodiesel production. Journal of Materials and Environmental Science 6(4): 1178-1185.
- Win SS, Trabold TA (2018b) Sustainable waste-to-energy technologies: Transesterification. Sustainable Food Waste-to-Energy Systems 89-109.
- Barabas I, Todoru IA (2011) Biodiesel quality, standards and properties. Biodiesel- Quality, Emissions and By-Products.
- Azeez AM, Fasakin AO, Orege JI (2019) Production, characterisation and fatty acid composition of jatropha curcas biodiesel as a viable alternative to conventional diesel fuel in Nigeria. Green and Sustainable Chemistry 9(1): 1-10.
- Nainggolan M, Sinaga A (2021) Characteristics of fatty acid composition and minor constituents of red palm olein and palm kernel oil combination. Journal of Advanced Pharmaceutical Technology and Research 12(1): 22-26.
© 2023 Charles Mazereku. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






