- Submissions

Full Text
Biodiversity Online J
Diversity and Ecology of phyllostomid Bat Species (Chiroptera: Phyllostomidae) in Caparaó National Park, Southeastern Brazil
Jonatas AT1*, Viviane SO2,3, Felipe C3, Ralphy LX3, Alessandro B2,3 and Graziele C4
1Programa de Pós-graduação em Biodiversidade e Saúde, Instituto Oswaldo Cruz, Rio de Janeiro, Brasil
2Universidade do Estado de Minas Gerais, UEMG, Carangola, Minas Gerais, Brasil
3Projeto Morcegos do Caparaó
4Universidade Iguaçu, Rio de Janeiro, Brasil
*Corresponding author:Jonatas Amorim Tavares, Instituto Oswaldo Cruz, Fundação Oswaldo Cruz, Rio de Janeiro, RJ, Brasil
Submission: August 19, 2022; Published: December 13, 2022

ISSN 2637-7082Volume3 Issue3
Abstract
Bats have varied feeding habits and have varied responses to habitat fragmentation and loss. In this study we analyzed the diversity and ecology of bat species in the understory of Parque Nacional do Caparaó, in the Atlantic Forest of Minas Gerais, southeastern Brazil. The captures were carried out from November 2011 to December 2013, with mist nets (9x3m), mounted at ground level, open at dusk and closed after six hours of exposure. A total of 288 individuals, six genera and seven species were captured. All species sampled belong to the Phyllostomidae family, in four subfamilies. Sternodematinae was the most abundant subfamily in the samples, representing 57% of the species and 76% of the specimens sampled. The composition of species diversity suggests that these disturbed fragments alter the dynamics of behavior and structure of these communities. Some bat species found in the PNC have an important association with the vertical stratum, making it evident that the structure and level of anthropization of the forest fragment is a factor that regulates the richness and abundance of bat species.
Keywords:Chiroptera; Atlantic forest; Species richness
Abbreviations:PNC-Caparao National Park; UEMG-State University of Minas Gerais
Introduction
The order Chiroptera is the most taxonomically diverse among mammals, with 1250 species, distributed in 20 families currently recognized in the world [1], representing 25% of mammals [2], with 15% of the total bat species having occurrence records for the national territory. Bats constitute a considerable part of the mammalian fauna in Neotropical environments and, frequently, have a higher species richness than the number of mammal species of other orders present in the same locality and region [3]. In Brazil, 180 species are recognized, with 98 occurring in the Atlantic Forest biome and 77 bat species recorded for the state of Minas Gerais [4-6]. Studies on the ecology of bats show that they are species responsible for interactions that are fundamental to the conservation and maintenance of ecosystems, controlling populations of invertebrates and vertebrates, dispersing seeds and pollinating flowers [7-9], in addition to being of public health interest [1]. According to Kalko [10], in an in-depth study of the ecology of Neotropical bats, found that there were species with strong specialization in diet and space use resulting from evolutionary adaptations in the morphology of their wings and in the frequency of echolocation, classifying them into ten different guilds [10]. As it is a very diverse group, each species uses the habitat according to its morphological, physiological and behavioral adaptations, with the phytophysiognomy of the place being a determining factor in its foraging area [11]. Many bat species inhabit similar environments, exhibiting similar foraging patterns, thus allowing them to be classified into groups that use closed habitats such as forests, grasslands, and areas above the canopy [12]. With increasing changes in natural ecosystems, continuous forest formations disappeared through degradation or were replaced by cities, plantations and pastures [13,14], with these changes and the fragmentation of forests, fragments have been isolated and possible foraging sites, generally exploited by bat species in search of resources [15].
As a result of these changes in the environment, many species are able to replace their natural habitats with similar ones [16,17], however, some species are sensitive to fragmentation, having in their behavior varied responses to this process [18], as reported by Schulze [19], who demonstrated that the species Sturnira lilium and Carollia perspicillata present different patterns in their behavior, foraging in the edges of anthropized forests in search of secondary food sources, having an increase in their populations, in addition to being good indicators of altered environments. [18], reported that several species of bats occupy different vertical strata of a forest, adapting to specific conditions in their behavior, as the species C. perspicillata, which forages from the forest to the understory, presenting changes in its diet and in the structure of its community due to the levels of disturbance and fragmentation of its niche. In order to evaluate the diversity and ecology of bat species in the understory in several areas of the Caparaó National Park, with different states of conservation and human disturbance, the objective of this study was to relate the diversity of these species to their ecology and food guild.
Material and methods
Study area
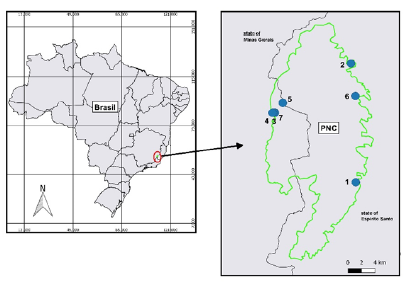
Located in Serra do Caparaó, the Caparaó National Park (PNC) located between the parallels 20°19’S and 20°37’S and the meridians 41°43’W and 41°53’W [20], is located in Serra do Caparaó on the border between two states, Minas Gerais and Espírito Santo, in southeastern Brazil, being a Federal Conservation Unit, with 31.8 thousand hectares, with 80% of its area located in the state of Espírito Santo (Figure 1). The region where the PNC is located has a mountain range that rises to 2800 m above sea level, forming the Caparaó Massif [21]. The climate according to Köppen is of the Cwb type: with dry winters and mild summers, being also characterized as altitude tropical, where the relief directly influences the temperature differences. The average annual temperature varies between 19 °C and 22 °C, with a maximum reaching 36 °C, and a minimum of -4 °C at the highest peaks. Average rainfall varies between 1,200 and 1,300mm per year [22] reaching 1,750mm in the northern region of the park [20]. The region was totally covered by Atlantic Forest Biome vegetation, with predominance of animal species adapted to the deformation of stony soils, intense cold, frost and formation of ice crusts [20]. With the increasing devastation and the loss of the forest for agriculture and livestock, much of its forests were destroyed, leaving the fauna small animals-such as opossum (Didelphis aurita Wied-Neuwied, 1826), cuíca (Philander opossum Linnaeus, 1758), paca (Cuniculus paca Linnaeus, 1766), tapeti (Sylvilagus brasiliensis Linnaeus, 1758), and caxinguelê (Guerlinguetus brasiliensis Thomas, 1901), and some predators such as the crab-eating fox (Cerdocyon thous Linnaeus, 1766), tayra (Eira barbara Linnaeus, 1758), raccoon (Procyon lotor Linnaeus, 1758) and a species of wildcat (Leopardus tigrinus Schreber, 1775); species threatened by fragmentation and hunting in the region [22]. Currently, it is possible to observe several plant formations in the Park, such as evergreen hygrophilous forests, seasonal subdeciduous forest, riparian forests, high altitude fields and rupestrian fields. This differentiation of vegetation formations was characterized by several factors such as climate variation, water courses, altitude, soil types, human interventions, deforestation and the introduction of exotic species [20,21]. The forest areas of the fragments surrounding the park are of secondary formation, altered by the action of fire, wood extraction and deforestation, leaving few preserved areas. The canopy of the forests varies between 20 and 30m, and plant species such as embaúbas (Cecropia spp.), quaresmeiras (Tibouchina spp., Miconia spp.), adragos (Croton spp. (Piptadenia spp.) [21].
Figure 1: Location of the Caparaó National Park, on the border of the states of Minas Gerais and Espírito Santo. Blue circles indicate the sampling points of bats from 2011 to 2013: 1- Santa Marta, 2- Pedra Roxa, 3- Vale Verde, 4- Estrada da Tronqueira, 5- Cachoeira Bonita, 6- Ponto do Cachorro, 7-Casa do Waldomiro.
Animal capture
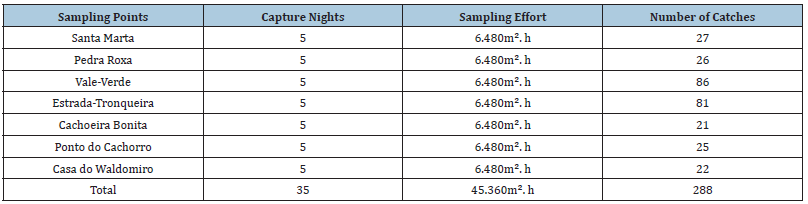
Captures were carried out in seven areas of the Caparaó National Park, in different seasonal periods, from November 2011 to October 2013, with five nights in each area (Table 1). The sampling effort was calculated according to [23]. All captures took place in the new and crescent phases of the moon, with temperatures ranging from 8.6 °C to 20.4 °C. Eight mist nets (9x3m) were used to capture bats, installed close to food sources, water bodies and flight routes, as well as existing trails in the forest and roads [24]. The nets were opened at dusk, reviewed at 15-minute intervals and closed after six hours of exposure [25]. The animals removed from the net were placed in cotton bags and taken to a field base for later identification of the species, biometrics, age class, sex and reproductive status. The age group was identified, following [26], by the ossification of the epiphyses of the metacarpals and phalanges of the forelimbs, with individuals classified as adults, juveniles and infants. On each sampling night, abiotic data (temperature, relative humidity and luminosity) were obtained and recorded at the sampling site. After screening, the bats were released at the same location. Species identification was based on [27], and taxonomic classification followed [28]. Sampling was carried out in accordance with the license issued by the Chico Mendes Institute for Biodiversity Conservation: SisBio 31547 (Chiroptera)-Alessandro Brinati. All specimens were handled following capture, handling and collection protocols defined by the American Society of Mammalogists [29]. The specimens are deposited at the Newton Baião de Azevedo Museum of Zoology at the Carangola Unit of the State University of Minas Gerais-UEMG.
Table 1:Sampling points, capture nights, sampling effort and number of individuals captured for bats in the Caparaó National Park from 2011 to 2013.
Result
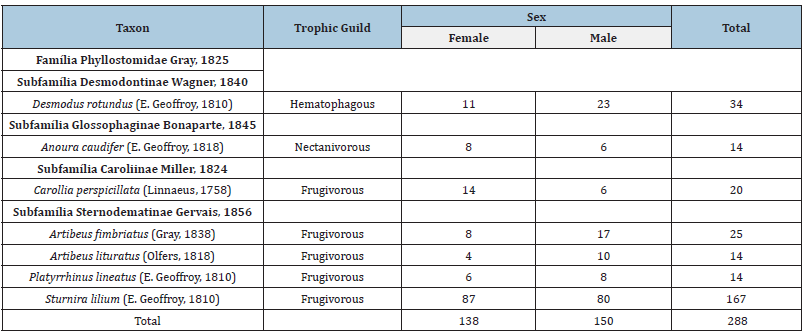
A total of 288 specimens were sampled, distributed in seven species, six genera, one family, in 35 nights of campaigns, with a sampling effort of 45.360m².h of net (Tables 1 & 2). All species sampled belong to the Phyllostomidae family, in four subfamilies. Sternodematinae was the most abundant subfamily in the samples, representing 57% of the species and 76% of the specimens sampled (Table 2). The species C. perspicillata was the most abundant, presenting a capture frequency of 58% in the samplings, being sampled at all points. Three trophic guilds were sampled, being a hematophagous species, a nectanivorous species and five frugivorous species. The predominance was of frugivorous bats, represented by 72% of the species (Table 2).
Table 2:Family, bat species, trophic guild, sex of individuals and total number of bats sampled in the Parque Nacional do Caparaó from 2011 to 2013.
Discussion
Our results showed 7% of the species recognized for the Atlantic Forest and 7% for the bat species registered for the state of Minas Gerais, these findings show an exclusive abundance of phyllostomid bats (Chiroptera: Phyllostomidae) for the PNC and all sampling sites, with a predominance of the S.lillium species with 58% of the individuals sampled. [30], relatam a plasticidade dessas espécies no uso e forrageio do ambiente, como a dominância nas comunidades de morcegos em áreas antropizadas e fragmentadas. A. fimbriatus, S. lillium and C. perspicillata showed a frequency of 73% in the samplings, being sampled at all points. The abundance of these species is directly related to the environmental history of the study area, because species that are more resistant to anthropization and that have plasticity in their diet are able to maintain larger populations compared to species that are sensitive to anthropization and environmental fragmentation, their diet being restricted and specialist [31]. Furthermore, they are common species for many AM fragments both in the state of MG and other states of MA [32-38]. The composition of catch diversity recorded in this work demonstrates a pattern of species richness and diversity compared to other studies carried out in preserved areas in Brazil, as seen in [39,40]. The high rate of specimens of the Stenodermatinae subfamily (A. lituratus, A. fimbriatus, P. lineatus, S. lilium) can be explained by the methodology used (gill nets in the understory), These animals are canopy and understory frugivores, with low foraging habits and are easily captured by the mist net, as observed in [15,41,42]. The abundance of the species S. lilium sampled in this study (58%), compared to other studies, shows that the species has a high frequency of capture in the understory, as observed by [39], who demonstrated that the fragmentation and disturbance of the environment directly affects the behavior of this species, and these responses are observed in its use and foraging in the environment. [43], related this behavior to the feeding guild of the species S. lilium, with the fruits consumed from low trees, such as Solanum crinitum, in relation to the forest canopy. [42], report that areas of fragmented and close vegetation on the plains help bats during their movement, forming great connections between the forest blocks of a forest [44], serving as a reference to locate themselves spatially when necessary, and protect themselves from predators and weather conditions [45,46].
Conclusion
The composition of the species diversity found in this study suggests that disturbed fragments alter the dynamics and structure of bat communities present in the locality [18,19]. Some bat species found in the PNC have an important association with floral composition, making it evident that the structure and level of anthropization of the forest fragment is a factor that regulates the richness and abundance of bat species.
Acknowledgement
JAT is grateful for the support of Parque Nacional do Caparaó and funding from Capes. VSO, AB thanks UEMG for its support and funding. FC, RLX thanks UEMG.
References
- Fenton MB, Simmons NB (2015) Bats, a world of science and mystery. Chicago pp: 303.
- Wilson DE, Reeder DM (2005) Mammals species of world: a taxonomic and geographic reference. Johns Hopkins University Press.
- Fenton MBL, Acharya D, Audet MBC, Hickey C, Merriman MK, et al. (1982) Foraging strategies of plant-visiting bats. New York pp. 287-325.
- Nogueira MR, Lima IP, Moratelli R, Tavares VC, Gregorin R, et al. (2014) Checklist of Brazilian bats, with comments on original records. Check List 10(4):808-821.
- Muylaert RL, Stevens RD, Esbérard CEL, Mello MAR, Garbino GST, et al. (2017) Atlantic bats: a dataset of bat communities from the Atlantic Forests of South America. Ecology 98(12): 3227-3227.
- Paglia AP, Fonseca GAB, Rylands AB, Herrmann G, Aguiar LMS, et al. (2012) Annotated checklist of brazilian mammals. Occasional Papers in Conservation Biology. Arlington.
- Gardner AL (1977) Feeding habits. pp: 293-350.
- Reis NR, Peracchi ALP, Lima IP, Pedro WA (2007) Morcegos do Brasil, Editora Universidade Estadual de Londrina, Londrina, Brazil.
- Kunz TH, Braun TE, Bauer D, Lobova T, Fleming TH (2011) Ecosystem services provided by bats. Annals of the New York Academy of Sciences 1223: 1-38.
- Kalko EK, Handley CO, Handley D (1996) Organization, diversity, and long-term dynamics of a neotropical bat community. Long-term studies of vertebrate Communities pp: 503-553.
- Kalko EKV, Handley CO (2001) Neotropical bats in the canopy: diversity, community structure, and implications for conservation. Plant Ecology 153: 319-333.
- Denzinger A, Schnitzler HU (2013) Bat guilds, a concept to classify the highly diverse foraging and echolocation behaviors of microchiropteran bats. Frontiers in Physiology 4: 1-15.
- Andrén H (1994) Effects of habitat fragmentation on birds and mammals in landscape with different proportions of suitable habitat: a review. Oikos 71: 355-366.
- Saunders DA, Hobbs RJ, Margules CR (1991) Biological consequences of ecosystem fragmentation: a review. Conservation Biology 5: 18-32.
- Bernard E, Fenton MB (2003) Bat mobility and roosts in a fragmented landscape in central Amazonia, Brazil. Biotropica 35: 262-277.
- Dixon MD (2012) Relationship between land cover and insectivorous bat activity in an urban landscape. Urban Ecosystems 15: 683-695.
- Estrada VS, Meyer CFJ, Kalko EKV (2010) Effects of tropical forest fragmentation on aerial insectivoros bats in a land-bridge island system. Biological Conservation 143(3): 597-608.
- Medellín RA, Equihua M, Amin MA (2000) Bat diversity and abundance as indicators of disturbance in Neotropical Rainforests. Conservation Biology 14: 1666-1675.
- Schulze MD, Seavy NE, Whitacre DF (2000) A comparison of the phyllostomid bat assemblages in undisturbed Neotropical forest and in forest fragments of a slash and burn farming mosaic in Peten, Guatemala. Biotropica 32(1): 174-184.
- IBDF (1981) Regulamento dos Parques Nacionais Brasileiros. Brasília pp: 12.
- Ibama (1995) Conhecimento Científico para Gestão Ambiental: Amazônia, Cerrado e Pantanal. Brasí
- Alvares CA, Stape JL, Sentelhas PC, de Moraes Gonçalves JL, Sparovek G (2013) Köppen's climate classification map for Brazil. Meteorologische Zeitschrift 22 (6): 711 728.
- Straube FC, BianconiI GV (2014) Sobre a grandeza e a unidade utilizada para estimar esforço de captura com utilização de redes de neblina. Chiroptera Neotropical 8(1-2): 150-153.
- Kunz TH, Parsons S (2009) Ecological and behavioral methods for the study of bats. Maryland pp: 901.
- Esbérard CEL, Bergallo HG (2008) Influência do esforço amostral na riqueza de espécies de morcegos no sudeste do Brasil. Revista Brasileira de Zoologia. 25(1): 67-73.
- Anthony ELP (1988) Age determination in bats. Washington pp: 47-58.
- Gardner AL (2008) Mammals of South America: Marsupials, xenarthrans, shrews, and bats. Chicago, USA, pp: 1: 360.
- Nogueira MR, Lima IP, Moratelli R, Tavares VC, Gregorin R, et al. (2014) Checklist of Brazilian bats, with comments on original records. Check List 10(4): 808-821.
- Sikes RS, Gannon WL (2011) Guidelines of the american society of Mammalogists for the use of wild mammals in research. J Mammal 97(3): 235-253.
- Andrade TY, Thies W, Rogeri PK, Kalko EKV, Mello MAR (2013) Hierarchiral fruit selection by neotropical leafnosed bats (Chiroptera: Phyllostomidae). Journal of Mammalogy 94: 1094-1101.
- Bolzan DP, Lourenço EC, Costa LM, Lins JL, Nogueira TJ, et al. (2010) Morcegos da região da costa verde e adjacências, litoral sul do estado do Rio de Janeiro. Chiroptera Neotropical 16(1): 586-595.
- Esbérard CEL (2003) Diversidade de morcegos em uma área de Mata Atlântica regenerada no sudeste do Brasil (Mammalia: Chiroptera). Revista Brasileira de Zoociências 35 (2): 189-204.
- Dias D, Peracchi AL, Silva SSP de (2002) Quirópteros do Parque Estadual da Pedra Branca, Rio de Janeiro, Brasil (Mammalia, Chiroptera). Revista Brasileira de Zoologia. 19(2):113-40.
- Faria MB, Ribeiro MCS, Oliveira ME de, Ferraz DS (2016) Estudo da quiropterofauna (Mammalia: Chiroptera) em duas reservas particulares do patrimônio natural da mata atlântica, minas gerais, Brasil. Boletin Sociedade Brasileira de Mastozoologia 77:117-23.
- Baptista M, Mello MAR (2001) Preliminary inventory of the bat species of the Poço das Antas Reserve, RJ. Chiroptera Neotropical 7(1-2): 133-135.
- Delciellos AC, Novaes RLM, Loguercio MFC, Geise L, Santori RT, et al. (2012) Mammals of Serra da Bocaina National Park, state of Rio de Janeiro, southeastern Brazil. Check List 8(4):675-692.
- Esbérard CEL (2006) Effect of collecting bats for nights in a row in the same place. Revista Brasileira de Zoologia 23 (4): 1093-1096.
- Esbérard CEL, Costa LM, Luz JL (2013) Morcegos de Morro de São João, Estado do Rio de Janeiro, Sudeste do Brasil. Bioscience Journal 29(2): 449-457.
- Pereira MJR, Marques JT, Palmerim JM (2010) Vertical stratification of bat assemblages in flooded and unflooded Amazonian forests. Current Zoology Beijin 56(4): 469.
- Tavares JA, Novaes RLM, Veríssimo I, Kuzel MAA, Costa NSF, et al. (2021) Bats from the Pedra Branca Forest, Rio de Janeiro, Brazil. Biodiversity Data Journal 9: e77400.
- Pedro WA, Taddei VA (1997) Taxonomic assemblage of bats from Panga Reserve, southeastern Brazil: Abundance patterns and trophic relations in the Phyllostomidae (Chiroptera). Boletim do Museu de Biologia Leitão 6: 3-21.
- Bernard E, Fenton MB (2007) Bats in a fragmented landscape: species composition, diversity and habitat interactions in savannas of Santarém, Central Amazonia, Brazil. Biological Conservation 134(3): 332-343.
- Norberg UM, Raynes JMV (1987) Ecological morphology and flight in bats (Mammalia; Chiroptera): wing adaptations, flight performance, foraging strategy and echolocation. Philosophical Transactions of the Royal Society of London B 316: 335-427.
- Frey EA, Bontadina F, Arlettaz R, Obrist MK (2013) Landscape connectivity, habitat structure and activity of bat guilds in farmland-dominated matrices. Journal of Applied Ecology 50: 252-261.
- Henry M, Pons JM, Cosson JF (2007) Foraging behavior of a frugivorous bat helps bridge landscape connectivity and ecological processes in a fragmented rainforest. The Journal of Animal Ecology 76: 801-813.
- Morris AD, Miller DA, Kalcounis RMC (2010) Use of forest edges by bats in a managed Pine Forest landscape. Journal of Wildlife Management 74(1): 26-34.
© 2022 Jonatas AT. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






