- Submissions

Full Text
Biodiversity Online J
Are Land Climatic Variables Good Predictors for the Distribution of Estuarine Microorganisms? A Study with the Microcrustacean Kalliapseudes Schubarti
Rômulo JR1,2* and Gustavo RL1,2,3
1Instituto da Biodiversidade-IBIO, Brasil
2Programa de Pós-graduação em Biologia Animal, Universidade Federal do Espírito Santo, Brasil
3Unidade de Medicina Tropical, Universidade Federal do Espírito Santo, Brasil
*Corresponding author: Rômulo José Ramos, Instituto da Biodiversidade-IBIO, Vila Velha, ES, Brasil.
Submission: December 06, 2021; Published: June 10, 2022

ISSN 2637-7082Volume2 Issue4
Abstract
The estuarine organisms present limitations in their distribution due to tolerance factors as salinity, sediment type, water temperature, among others. Kalliapseudes schubarti is common in estuaries and slimy plains of the Brazilian south and southeast regions, where it plays an important role in the food chain. Recently, this species was found in areas where its distribution was unknown, as in the county of Guarapari, state of Espírito Santo (ES), Brazil. This occurrence may be suggesting that K. schubarti presents a wider distribution than the expected. Based in this found, by means of ecological niche modeling, we determine its potential distribution, using climatic variables. The first scenario, without ES data, showed that K. schubarti presents distribution from state of Rio Grande do Sul (RS) to the state of Rio de Janeiro. The second scenario, using the ES data, showed that its distribution ranges from RS to the south of state of Bahia (BA). We suggest the Brazilian coast temperatures are not a limitation factor for this species, which occurs in estuarine regions of soft bed rich in organic substance. This sediment, common in the some regions of ES and BA, should be the main factor for the K. schubarti occurrence.
Keywords:Estuaries; Geographical distribution; Bioclimatic parameters; Genetic algorithms; Brazillian coast
Introduction
Estuaries lie at the interface between marine, freshwater, terrestrial and atmospheric systems, and thus comprise some of the most dynamic ecosystems on earth, supporting a rich variety of plants and animals. They also provide foci for human activities and are amongst the most anthropogenically degraded of all ecosystem types (Edgar et al. 1999). The study of the species of these environments is the base to understand its bioecology and also to infer about several kinds of natural effects and also those caused by antropic interventions. This serves as subsidy for biodiversity conservation (Fairweather, 1999). Members of the crustacean order Tanaidacea are commonly found in marine and brackish-water environments, where they occupy a variety of benthic habitats over a wide range of depths (Hassack & Holdich, 1987). This group is typically represented by marine forms, however, with most of the 700 known species having been reported in deepwater environments. Although relatively few species live in marine coastal habitat [1], they form a significant groupespecially in soft bottoms [2]. Kalliapseudes schubarti Mañé-Garzon, 1949 (Tanaidacea, Kalliapseudidae) is a common species in estuaries and slimy plains along the southeast and southern Brazil, Uruguay and north Argentina coasts [3]. It is a benthonic species that uses as habitat the nonconsolidated sediments of the subtidal soft bottom environment, in which it constructs tubes and through filtration acquires food that is based on detritus and plankton. Its size is between 7 and 10mm, and due to this species be a part of fish and crustaceans’ diet, it plays an important role in the food chain [4].
Recently, K. schubarti was collected by (Costa & Nalesso, 2006) in the municipality of Anchieta, state of Espírito Santo, Brazil. This record was a considerable extension of its known distributionabout 2º in latitude to the north. This finding might suggest that this species presents a geographic distribution even wider than the expected. According to [5], the way the environment influences its abundance and distribution is not well understood. In general, estuarine organisms present limitation in distribution due to variations in salinity, sediment type, water temperature, among others (Muniz & Venturini, 2001). Because of some estuary characteristics (e.g., salinity and water temperature) can be strongly influenced by land climatic variables (e.g., precipitation and temperature) (Dunton et al. 2001), these land variables might also affect estuarine organismseven limiting its distribution (Costa & Nalesso, 2006; Colling et al. 2007). Consequently, it is important to determine the role of land climatic variables on estuaries and on its organisms. It is possible to predict a species distribution based on its ecological requirements using an approach to increasing the utility of available distributional data for widely sampled regions extrapolating from known points to unknown areas [6]. This approach centers on estimating the dimensions of species ecological niches-the ecologic space within which a species can maintains populations without immigration [7]. These models have been employed in various ways, such as in studies of species geographical distribution, effects of environmental variables and future climate variation on species distribution, habitat selection, better design of reserves for rare and endemic species or of priority areas for conservation [6,8,9,10]. We them addressed the following question and tried to answer it using an ecological niche modeling approach: are land climatic variables good predictors for the distribution of estuarine microcrustacean K. schubart? If the answer be yes, the models will help us to find new suitable areas for its occurrence in southern Atlantic coast of the Neotropics. The answer will also help in understanding the role of land climatic variables on estuarine organisms. This study is a novel in ecological niche modeling of species in estuaries. We emphasize that the abundance of a species in a region might determine if it will be rare and consequently difficult to be found. and the lack of knowledge about its distribution might be due to the restricted areas sampled in these regions [11-13].
Materials and Methods
Occurrence data
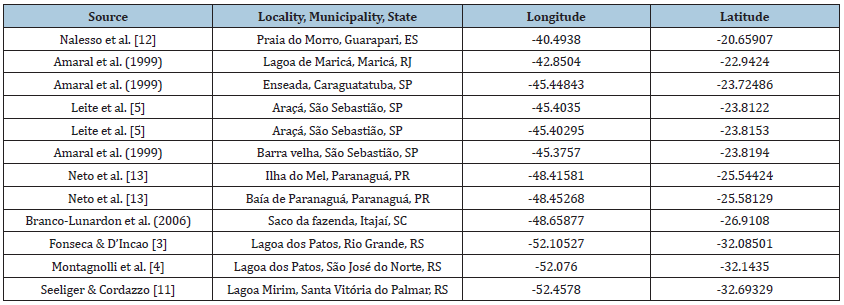
We obtained the occurrence records of K. schubarti from a vast literature survey, being each occurrence point showed in Table 1.
Table 1: Occurrence data of Kalliapseudes schubarti Mañé-Garzon, 1949.
Environmental variables
We obtained the 19 bioclimatic data layers, accurate to 0.042 degrees, used in the modeling from the land coastal geographic environmental data of World Clim database. We used the following variables: temperature seasonality, maximum temperature of warmest month, minimum temperature of coldest month, mean temperature of warmest quarter, mean temperature of coldest quarter, annual precipitation, precipitation seasonality, precipitation of wettest month and precipitation of driest month. Considering the effect of precipitation on coastal salinity and the environment temperature on water temperature, we have chosen these particular variables because of the importance of temperature and salinity on species mortality rate [3] and the extreme condition from environment seems to be an important factor that determines species distribution (Parsons 2005).
Modeling approach
We used the Genetic Algorithm for Rule-Set Prediction (GARP) for modeling the distribution of K. schubarti. This algorithm uses known occurrence points and digital maps of relevant ecological dimensions to produce a model of a species ecological niche, which when projected onto a landscape provides a prediction of the species geographical distribution [14,15]. We modeled two scenarios for K. schubarti: in the first, we used 11 occurrence points of K. schubarti, of which 9 had been considered geographically distinct and used for construction of the models; and for the second scenario, we used 12 occurrence points of K. schubarti, including the new record from municipality of Guarapari, being 10 of then considered geographically distinct. Then, we defined the program to run 2000 iterations in 50 generations, with convergence criterion of 0.01. We used the function Best Subset to select only the ten models with best accuracy of prediction, being removed the models with more than 10% of extrinsic omission [16]. We had imported the ten models in the Geographic Information System ArcView 3.2a [17], and to create the potential distribution maps, we summed the ten selected models, following [6]. This procedure allows to be representing in a map of the degree of likelihood of occurrence of species in a unit grid based on the number of models that predicted its presence there.
Results
Based on the 50% subsets of points set aside before modeling
to test, the GARP models for the scenarios were highly significant
statistically. Indeed, compared with random models, the probability
of such high predictivity was very low (x2 tests, 3.3−8
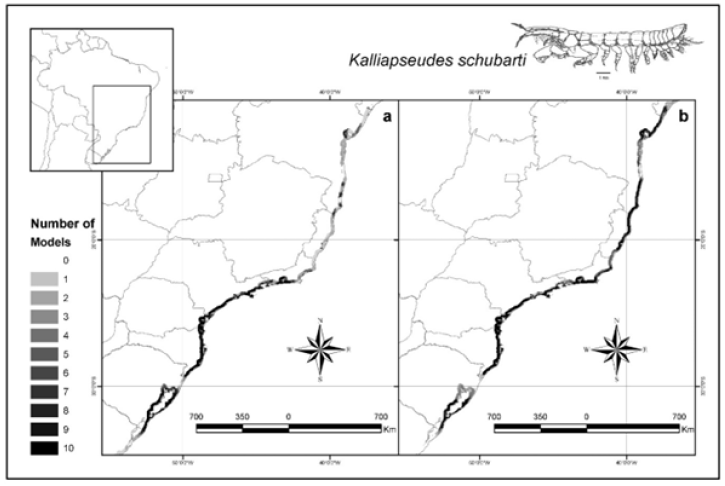
Figure 1: Modeled distribution of Kalliapseudes schubarti in the southern neotropical Atlantic coast. With the
occurrence data of the state of Espírito Santo removed
a) With the occurrence data of the Espírito Santo included
b) The K. schubarti illustration was obtained from Montagnolli et al (2004).
Discussion
The distribution of K. schubarti observed in the Figure 1a suggests that this crustacean presents a distribution restricted to temperate/hot transitional regions, in according to [18], correspondent to the states of Rio de Janeiro, São Paulo, Paraná, Santa Catarina and Rio Grande do Sul. However, the ecologic modeling with the inclusion of occurrence record of K. schubarti in Guarapari region, south of the Espírito Santo, located in a tropical area, has showed a new scenario (Figure 1b), in which the distribution of this crustacean was not restricted to temperate/ hot transitional localities, suggesting that the Brazilian coast temperatures are not a limitation factor for this species [18] found population increase in periods of spring and summer in São Paulo, and [19] found, in the autumn and winter, the largest densities of this crustacean (about 12,808 individuals for square meter) in the estuarine areas of Lagoa dos Patos in Rio Grande do Sul, suggesting that low temperatures are not a limitation factor for the occurrence of this crustacean. According to [5], this tan aid occupies soft bed estuarine regions that are especially rich in organic matter, as found in the estuary of Lagoa dos Patos [19], suggesting that the sediment can be more determinative in the establishment of the specie in other regions. Considering the results of our work, and that great part of the Brazilian coast presents suitable substrata for the establishment of this species, soil with rich soft unconsolidated substrata brought from the mouth of a river and lakes [20], we suggested that this species should present a wider distribution than the registered, and the lack of records is not related to the nonexistence of the species but to the lack of sampled regions and identifications of the K. schubarti in some regions of the Brazilian coast. Despite our results, we need to acquire better environmental data from the ocean coast in course to generate more precise distributional models. The use of environmental database of better quality can still turn better the result of modeling [21-27].
References
- Pires AMS (1980) Ecological studies on intertidal and infralitoral Brazilian Tanaidacea (Crustacea , Peracarida). Stud Neotr Faun Env 15(3-4): 141-153.
- Leite FPP (1995) Temporal and spatial distribution of Kalliapseudes schubarti Mañé-Garzon, 1949 (Crustacea, Tanaidacea) in the Araçá region, São Sebastião (SP). Arq Biol Tecnol 38(2): 605-618.
- Fonseca DB, Incao DF (2006) Mortality of Kalliapseudes schubartii in unvegetated soft bottoms of the estuarine region of the Lagoa dos Patos. Braz arch biol technol 49(2): 257-261.
- Montagnolli W, Zamboni A, Luvizotto SR, Yunes JS (2004) Acute effects of Microcystis aeruginosa from the Patos Lagoon estuary, southern Brazil, on the microcrustacean kalliapseudes schubartii (Crustacea: Tanaidacea). Arch Environ Contam Toxicol 46(4): 463-469.
- Leite FPP, Turra A, Souza ECF (2003) Population biology and distribution of the Tanaid Kalliapseudes schubarti Mañé-Garzon, 1949, in an Intertidal flat in southeastern Brazil. Braz J Biol 63(3): 469-479.
- Peterson AT, Ball LG, Cohoon KP (2002) Predicting distribution of Mexican birds using ecological niche modelling methods. Ibis 144: 27-32.
- Hutchinson GE (1957) Population Studies-Animal Ecology and Demography-Concluding Remarks. Cold Spring Harbor Symposia on Quantitative Biology 22: 415-427.
- Anderson RP, Laverde M, Peterson AT (2002) Using niche-based GIS modeling to test geographic predictions of competitive exclusion and competitive release in South American pocket mice. Oikos 98(1): 3-16.
- Peterson AT, Soberon J, Sánchez CV (1999) Conservatism of ecological niches in evolutionary time. Science 285(5431): 1265-1267.
- Peterson AT, Vieglais DA (2001) Predicting species invasions using ecological niche modeling. BioScience 51(5): 363-371.
- Seeliger U, Cordazzo CV (1993) Estuário da Lagoa dos Patos e costa adjacente.
- Nalesso RC, Paresque K, Almeida LG, Piumbini PP, Nickel V (2005) Influence of mytilculture on the sediment benthic community of Praia do Coqueiro, Anchieta, ES - Phase II. II Brazilian Congress of Oceanography, Vitória, ES, Brazil.
- Neto JCF, Krul R, Lana PC, Camargo M (2002) Parque Natural Municipal do Manguezal do Rio Perequê: Plano de manejo. Brasil.
- Stockwell DRB, Noble IR (1992) Induction of sets of rules from animal distribution data: A robust and informative method of analysis. Math Comp Simul 33(5-6): 385-390.
- Stockwell DRB, Peters D (1999) The GARP modelling system: Problems and solutions to automated spatial prediction. Int J Geogr Inf Sci 33(2): 143-158.
- Anderson RP, Lew D, Peterson AT (2003) Evaluating predictive models of species’ distributions: criteria for selecting optimal models. Ecol Model 162(3): 211-232.
- Esri (1999) Arc View GIS 3.2a. Environmental Systems Research Institute. USA.
- Seeliger U, Odebrecht C, Castello JP (1998) The Coastal and marine ecosystems of the extreme South of Brazil. Editora Ecoscientia pp: 326.
- Amaral ACZ, Amaral EHM, Leite FPP, Gianuca NM (2000) Avaliação e ações prioritárias para a conservação da biodiversidade da zona costeira e marinha: Diagnóstico sobre praias arenosas. Bases De Dados Tropicais (BDT).
- Amaral ACZ, Mac Cord FS, Borges M, Rizzo AE (2005) Macroinvertebrados bentônicos de fundos inconsolidados. Programa Biota, ES, Brasil.
- Lunardon BMJ, Branco JO, Verani JR (2000) Relações tróficas entre macroinvertebrados e peixes, na Armação do Itapocoroy. Editora da UNIVALI. p. 183-196
- Costa J, Peteron AT, Beard CB (2002) Ecologic niche modeling and differentiation of populations of Triatoma brasiliensis Neiva, 1911, the most important chagas disease vector in northeastern Brazil (Hemiptera, Reduviidae, Triatominae). Am J Trop Med Hyg 67(5): 516-520.
- Hijmans RJ, Cameron JL, Parra JL, Jones PG, Jarvis A (2004) The WorldClim interpolated global terrestrial climate surfaces.
- Peterson AT, Ortega HMA, Bartley J, Sanchez CV, Soberon J, et al. (2002) Future projections for Mexican faunas under global climate change scenarios. Nature 416: 626-629.
- Peterson AT, Stockwell DRB, Kluza DA (2002) Distributional prediction based on ecological niche modeling of primary occurrence data. Island Press pp: 617-623.
- Peterson AT (2001) Predicting species distributions based on ecological niche modeling. Condor 103: 599-605.
- Rocha DC, Lima PCWC, Santos CSG, Lana PC, Camargo MC (2005) Impacto de atividades antrópicas sobre o macrobentos da Baía de Paranaguá (Paraná). II Congresso Brasileiro de Oceanografia, Vitória Brasil.
© 2022 Rômulo JR. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






