- Submissions

Full Text
Biodiversity Online J
Pollinator Diversity and Abundance in a Pea Crop (Pisum Sativa L.) from Valladolid (Spain)
Aguado-Martín LO1*, Miranda-Barroso L2, Gugger R3 and Peris-Felipo FJ3
1Andrena Initiatives and Environmental Studies, Spain
2Sustainable Agriculture Syngenta Spain, Spain
3Syngenta Crop Protection AG, Switzerland
*Corresponding author: Luis Oscar Aguado-Martín, Andrena Initiatives and Environmental Studies, Nueva del Saliente 1-Bis, 47328 Valladolid, Spain.
Submission: December 10, 2021; Published: February 23, 2022

ISSN 2637-7082Volume2 Issue1
Abstract
The present work assesses the diversity of pollinators in a pea (Pisum sativum L) crop from La Overuela (Valladolid, Spain). It is based on samplings carried out in 2008 that combined visual observations and the use of sweeping nets. The field work identified a total of 317 individuals, belonging to 25 genera and 42 species. The most abundant species discovered are Eucera codinai Dusmet and Alonso, 1926 with 46.68% and Xylocopa violacea (L. 1758) with 19.87% of abundance. Moreover, the phenological analysis clearly confirms there is a relationship between plant growth stages and the presence of pollinators in a crop commonly considered to be self-pollinating. This demonstrates the important role that pollinators play in pea cross-pollination.
Keywords: Pollinators; Natural enemies; Bees; Wild bees; Pea; Pisum sativum; Agriculture; Spain
Introduction
Pea - Pisum sativum L. (Fabacea)- is grown in most parts of the world. After soybean and
beans, it is the third most significant legume grain [1]. In Spain, over 14,000 hectares of peas
are cultivated, and its total production is almost 104,000 tons [2]. Pea plants are very resistant
in case of drought and are quite undemanding in terms of soil quality [3]. As pea fixes
nitrogen, it is considered very beneficial for land treatment and a good forerunner to the next
crop. Pea is also used to feed livestock, to make hay and as a manure to fertilize soil [4,5]. Peas
stand out for their high protein content (20–27% on average), as well as their high levels of
manganese, vitamin C (antioxidant), vitamin K (important for bone health), vitamin B1 or
thiamin (essential for growth), vitamin B9 or folic acid (formation of structural proteins and
hemoglobin) and fiber (promotes intestinal transit). The pea flower is a classical papilionaceous
formed by the union of five petals [6]. Pea flowers produce nectar that attracts many
insects, mainly bee species [7,8]. Peas self-pollinate before the flower opens [9,10]. This form
of reproduction also reduces ecological risks that could result from potential trans genetic
migration via pollen to related wild species or other cultivars, if genetically engineered peas
were to be approved for farming in the future [11,12].
However, Govorov [13] observed a cross-pollination rate of about 25% in peas. More recently,
several studies have shown that the percentage may reach up to 28.57% under certain
conditions [14]. Despite such research, knowledge about pea pollinators is very low; there are
only few studies so far [5,15,16]. The present work examines the diversity and abundance of
pollinator entomofauna in the pea crop. It was undertaken in the field station of the Agrarian
Technological Institute of Castilla y León (Valladolid; Spain).
Material and Methods
Area of study
The research was carried out at the Finca Zamadueñas of the ITACyL (Agrarian Technological
Institute of Castilla y León), in La Overuela (Valladolid, Spain; 41°42’09.1”N 04°42’23.0”W). The farm has a slightly continental Mediterranean
climate with hot, dry, and short summers. Winters are very cold and
partially cloudy. Annual rainfall is approximately 490mm. [17]. The
crop covered in our study is pea (Pisum sativum L. 1753), which was
planted in a standard-conventional design. The field size was six
hectares. During the study, the agricultural practices such as fertilization
and phytosanitary treatments remained unchanged (Figure
1).
Figure 1: Pea crop field view and location.

Experimental design and data analysis
Insect sampling was carried out during the flowering period of the pea crop. Given the location, this was from 6th May to 27th June 2008. During this period, to characterize the main pollinators, the crop was visited every four days, which resulted in a total of 14 field visits. Insect abundance was assessed by combining visual observations and the use of sweeping nets (observed and captured specimen numbers were merged to perform the corresponding analyses). The observations were done by moving in a zigzag along fixed transects of 50x2m during 15 minutes per line and one hour per day. Time intervals between the visits differed, they took place both on sunny and cloudy days. The collected specimens are preserved in cyanide to keep them intact and to avoid discoloration. All specimens were identified to species level using appropriate entomological literature [18-23] and have been deposited at the Andrena Entomological Collection (Valladolid, Spain; AECV). Meteorological data was collected by the meteorological station located in La Overuela (Valladolid) [17].
Results and Discussion
Diversity of insects
During the sampling period, 317 individuals belonging to three orders (Coleoptera, Diptera and Hymenoptera), 25 genera and 42 species were collected (Table 1). The most abundant genus was Eucera Scopoli, 1770 with 158 individuals (49.84%) followed by Xylocopa Latreille , 1802 with 71 individuals (22.39%). Similar results in pigeon pea (Cajanus cajan (L.) Millspaugh) were observed in previous studies. Otieno et al. [16] collected 1008 visitors from 31 bee genera in 49 days, with Megachile Latreille , 1802 being the most abundant genus (28.57 %). Li et al. [15] observed insect pollinators in two different locations and found 46 and 25 species in only two days.
Table 1: List and abundance of the species collected in the field of peas by sampling day. COL: Coleoptera; DIP: Diptera; HYM: Hymenoptera. Cells in green specify the number of individuals detected; cells in white indicate their absence.
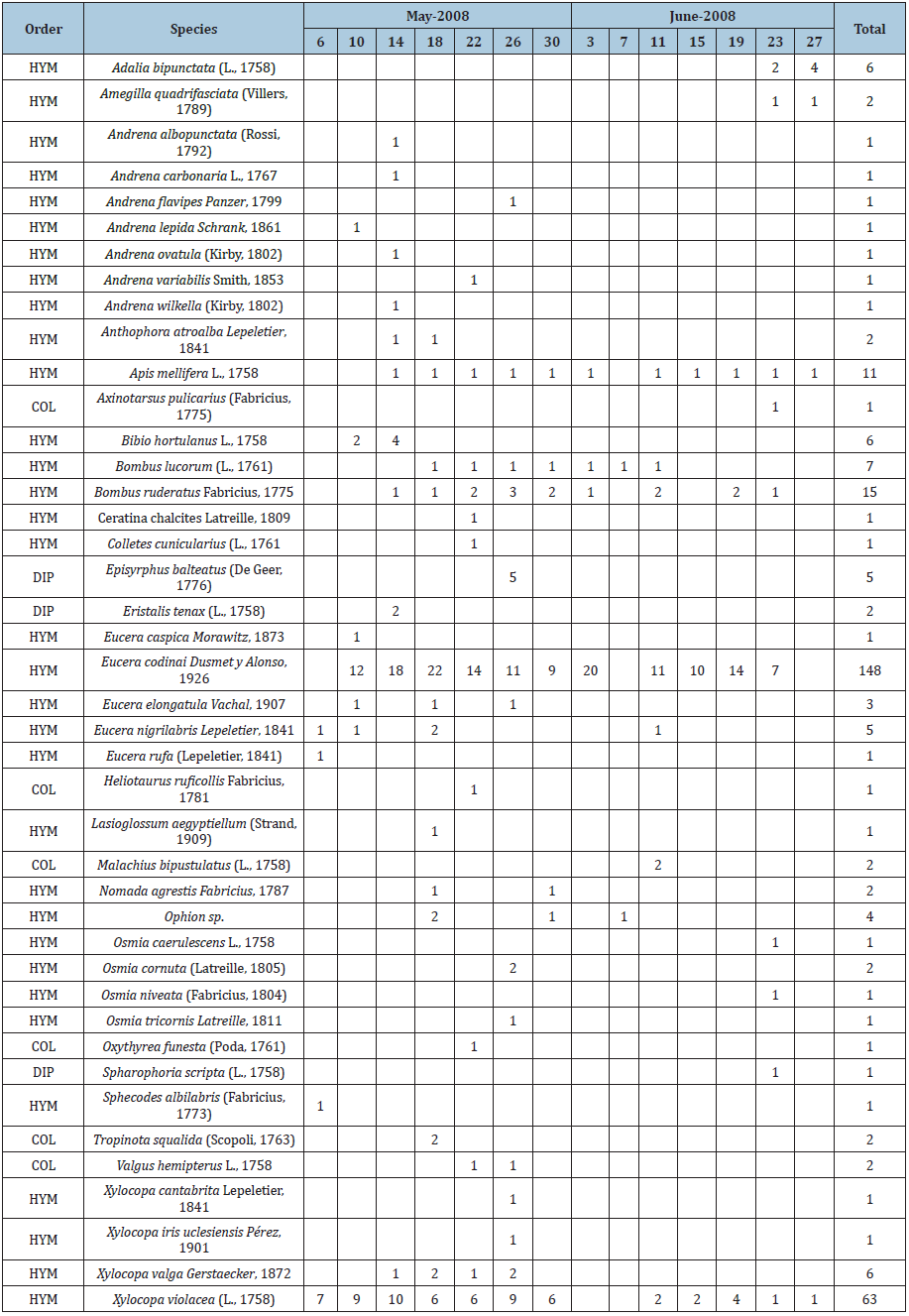
Pea crop pollinators
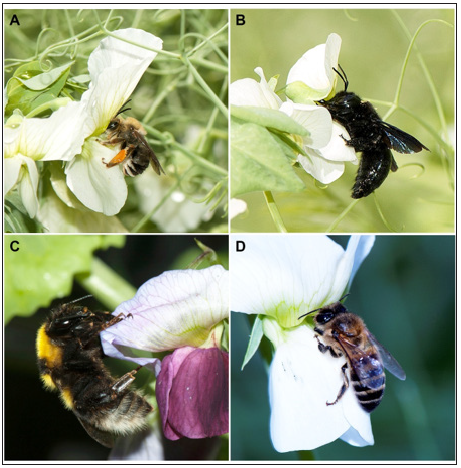
The specific analysis showed that Eucera codinai Dusmet and Alonso, 1926 (Figure 2A) with 148 individuals (46.68%) and Xylocopa violacea (L. 1758) (Figure 2B) with 63 (19.87%) were the most abundant species, with 66.55% of the total abundance, followed by Bombus ruderatus (Fabricius, 1775) (Figure 2C) with 15 individuals (4.73%) and Apis mellifera L. 1758 (Figure 2D) with 11 (3.47%). These results, in combination with the conclusions drawn by Otieno et al. [16], show the strongest relationship between polli nation and the carpenter bee (Xylocopa spp.), social bee and solitary bee guilds, which are among the most abundant bees visiting pea flowers in this system. However, they differ from those obtained by Naeem et al. [5] in Peshawar (Pakistan), where the most abundant pollinator species identified was the marmalade hoverfly (Episyrphus balteatus (De Geer, 1776)) with 35.22%, while in our case study we only found five individuals (1.57%) of this species. The presence of these species is closely linked to their morphology and the morphology of the pea flower. As a matter of fact, the size of the pea flower, its keel that is difficult to open, and its heavy pollen are indispensable details to consider when trying to understand the pollinators selection [8,24-26]. Indeed, insects must be very robust, so they can open the keel. In addition, they must have a long tongue to reach nectar and perform a vibration with their body called buzz pollination by which they shake the flowers and achieve efficient pollination [8,27,28]. Eventually, their body must be fit for transporting considerable loads of pollen while flying from one flower to the next [8]. Eucera codinai and Xylocopa violacea perfectly fit these criteria. Eucera’s body is longer than 1.5cm, it has a wingspan of more than 2.2cm and a long tongue. The body of Xylocopa is 2.5-3cm long, its wingspan measures 4-5cm and it also has a long tongue [8,29-31]. To the contrary, the individuals of Apis mellifera are not suitable to this type of pollination service given their lack of sufficient weight and strength [32]. Ortiz-Sánchez & Aguirre-Segura [33] determined that the dry weight of Xylocopa violacea is 268.3mg, compared to 20.2mg only for A. mellifera. These results were reinforced by Campbell et al. [34] who analyzed the number of pollen grains in different species and observed that species of the genus Xylocopa generally transported around 856.000 grains while A. mellifera was able to only carry approximately 2.800 pollen grains.
Figure 2: Most abundant bees. A. Eucera codinai Dusmet and Alonso, 1926 B. Xylocopa violacea (L. 1758) C. Bombus ruderatus (Fabricius, 1775) D. Apis mellifera L. 1758.
Flower-pollinator relationship
Seasonal patterns related to flower blossoming and insect abundance could be discerned. The full dataset shows that pollinators were visiting the crop throughout its entire flowering period. Species such as E. codinai and Xylocopa violacea were found on 11 and 12 days respectively, while Bombus ruderatus was observed on nine days (Table 1). The phenological analysis (Figure 3) clearly shows the relationship between plant growth stages and the presence and abundance of insects. As a matter of fact, pea flowers begun to open on 6th May. During the first two weeks, there was an evident increase in individuals present in the crop. On our field visits, we observed an average of 39 individuals for almost three weeks (until 26th May). After that date, the number of individuals decreased to a stable average of 18 individuals until the end of the flowering period. This decrease is clearly linked to the progressing pollination. Indeed, after one month, most of the flowers had already been pollinated. Rainy weather was the cause for the low level of individuals (2) collected on 7th June [17]. Correlating insect abundance and temperature levels shows that abundance is highest when temperatures are between 18-26 °C. These results coincide with previous research that identified temperatures from 15 to 25 °C and a relative humidity of 50-75% as ideal for insect flight and good pollination [8,35].
Figure 2: Relationship between climatic conditions (temperature) and the phenology and number of pollinators in pea crops.

Conclusion
This research proves that there is a great diversity and abundance of pollinating insects present in pea fields. It demonstrates the important role they play in pea cross-pollination. It also prepares the ground for future studies to examine and assess the overall impact of cross pollination and how it affects the genetic characteristics of Pisum sativum L. (Fabacea).
Acknowledgement
We are very thankful to Leopoldo Castro for his kindness and support during our research.
References
- Timmerman VGM, Mills A, Whitfield C, Frew T, Butler R, et al. (2005) Linkage mapping of QTL for seed yield, yield components, and developmental traits in pea. Crop Science 45(4): 1336-1344.
- MARM Ministry of Agriculture, Fisheries and Food (2019) Agri-food Statistics.
- Arnadillo N (2013) Agronomic and physiological knowledge of tear pea production under Guipúzcoa conditions. Establishment of the bases of its management for the improvement of quality. Universidad Pública de Navarra, Spain, p. 41.
- Cherepanov SK (1995) Plantae vasculares rossicae et civitatum collimitanearum (in limics ussr olim). List of vascular plants of russia. St. Petersburg: Mir I Semia, Russia, p. 990.
- Naeem S, Ahmad S, Sohail K, Dad R, Shah B (2016) Insect pollinators and their relative abundance on pea (Pisum sativum) at Peshawar. Journal of Entomology and Zoology studies 4(1): 112-117.
- Sihag RC (1988) Characterization of the pollinators of cultivated cruciferous and leguminous crops of sub-tropical Hissar, India. Bee World 69(4): 153-158.
- Kevan PG, Phillips T (2001) The economics of pollinator declines: assessing the consequences. Conservation Ecology 5: 8.
- Aguado LO, Viñuelas E, Ferreres A (2016) Guide to pollinators of the iberian peninsula and the balearic and canary Archipelagos. Mundiprensa & Syngenta Editions Madrid, Spain, p. 364.
- Cooper DC (1938) Embriology of Pissum sativum. Botanical Gazette 100: 123-132.
- Warnock SJ, Hagedorn DJ (1954) Stigma receptivity in peas (Pisum sativum). Agronomy Journal 46(6): 274-277.
- Polowick PL, Vandenberg A, Mahon JD (2002) Field assessment of outcrossing from transgenic pea (Pisum sativum) plants. Transgenic Research 11(5): 515-519.
- Kosterin OE, Bogdanova VS (2014) Efficiency of hand pollination in different pea (Pisum) species and subspecies. Indian Journal of Genetics 74(1): 50-55.
- Govorov LI (1928) Pea of Afghanistan (To the problem of the origin of cultivated peas). Proceedings on Applied Botany, Genetics and Select 19: 497-522.
- Bogdanova VS, Berdnikov VA (2000). A study of potential ability for cross-pollination in pea originating from different parts of the world. Pisum Genetics 32: 16-17.
- Li Z, Liang N, Hong M, Saxena KB, Liu X, et al. (2012) Insect pollinators in CGMS hybrid seed production of Cajanus cajan. Acta Agronomica Sinica 37(12): 2187-2193.
- Otieno M, Sidhu CS, Woodcock BA, Wilby A, Vogiatzakis IN, et al. (2015) Local and landscape effects on bee functional guilds crops in Kenya. Journal of Insect Conservation 19: 647-658.
- (2021) AEMET Meteorology Statal Agency, Spain.
- Amiet F, Herrmann M, Müller A, Neumeyer R (2001) Fauna Helvetica APIDAE 3: Halictus, Lasioglossum; Center suisse de cartographie de la faune (CSCF Info Fauna) Swiss Entomological Society (SEG/SES), Bern, Switzerland, p. 211.
- Amiet F, Hermann M, Müller A, Neumeyer R (2007) Fauna Helvetica APIDAE 5: Ammobates, Ammobatoides, Anthophora, Biastes, Ceratina, Dasypoda, Epeoloides, Epeolus, Eucera, Macropis, Melecta, Melitta, Nomada, Pasites, Tetralonia, Thyreus, Xylocopa. Center Suisse de cartographie de la faune (CSCF Info Fauna) Swiss Entomological Society (SEG/SES), Bern, Switzerland, p. 181.
- Amiet F, Hermann M, Müller A, Neumeyer R (2010) Fauna Helvetica APIDAE 6: Andrena, Meliturga, Panurginus, Panurgus. Center suisse de cartographie de la faune (CSCF Info Fauna) Swiss Entomological Society (SEG/SES), Bern, Switzerland, p. 316.
- Oosterbroek P (2006) The European families of the diptera. Identification, Diagnosis, Biology. KNNV Publishing: Utrecht, Netherlands, p. 206.
- Scheuchl E (2000) Illustrated identification tables of wild bees. Volume I: Anthophoridae. Preisinger KG, Landhut, Germany, p. 158.
- Scheuchl E (2006) Pocket dictionary of wild bees in Germany and Austria. Volume I: Megachilidae-Melittidae. Lito Tryk A/S, Berlin, Germany, p. 192.
- Mackie WW, Smith FL (1935) Evidence of field hybridization in beans. Journal of the American Society of Agronomy 27(11): 903-909.
- Pouvreau A (2004) The pollinating insects. Delachaux and Niestle Paris, p. 189.
- Aguado MLO (2014) Study of the entomogamous pollination of the pea (Pisum sativum) and its pollinators. XI Annual Scientific Meeting ECOFLOR (Thematic Network of Ecology and Floral Evolution), Puerto Real (Cadiz).
- Arroyo MT (1981) Breeding systems and pollination biology in Leguminosae. In: Polhill RM, Raven PH (Eds.), Advances in legume systematics 2: 723-769.
- Aouar SM, Louadi K, Doumandji SE (2008) Pollination of the broad bean (Vicia faba L. var. major) (Fabaceae) by wild bees and honeybees (Hymenoptera: Apoidea) and its impact on the seed production in the Tizi-Ouzou area (Algeria). African Journal of Agricultural Research 3(4): 266-272.
- González LJR (2021) Xylocopa violacea (Linnaeus, 1758). p. 479.
- Goulet H, Huber JT (1993) Hymenoptera of the world: An identification guide to families. Centre for Land and Biological Resources Research. Ottawa Ontario Research Branch Agriculture, Canada, p. 680.
- Michener CD (2007) The Bees of the world. Second Edition, The John Hopkins University Press, United States of America p. 953.
- Marzinzig B, Brünjes L, Biagioni S, Behling H, Link W (2018) Bee pollinators of faba bean (Vicia faba L.) differ in their foraging behaviour and pollination efficiency. Agriculture Ecosystems and Environment 264(1): 24-33.
- Ortiz SFJ, Aguirre SA (1992) Comparison of the effectiveness of different heights in capturing bees using moericke traps (Hymenoptera, Apoidea). Graellsia 48: 35-43.
- Campbell JW, Irvin JH, Ellis JD (2018) Bee contribution to partridge pea (Chamaecrista fasciculata) pollination in Florida. The American Midland Naturalist Journal 179(1): 86-93.
- Hormaza I (2018) Importance of pollination for productivity in subtropical fruit trees. Institute of Subtropical and Mediterranean Horticulture & CSIC, p. 118.
© 2021 Aguado-Martín LO. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






