- Submissions

Full Text
Biodiversity Online J
The Andean Lupine-‘El Chocho’ or ‘Tarwi’ (Lupinus mutabilis Sweet)
Doris Chalampuente-Flores1,2, César Tapia Bastidas2 and Marten Sørensen3*
1University Santiago de Compostela, Spain
2Technical University of the North, Ecuador, National Institute of Agricultural Research, Ecuador
3Department of Plant & Environmental Sciences, University of Copenhagen, Denmark
*Corresponding author: Marten Sørensen, Department of Plant & Environmental Sciences, University of Copenhagen, Denmark
Submission: April 24, 2021; Published: June 17, 2021

ISSN 2637-7082Volume1 Issue4
General Observations on the Culture of Lupine (Lupinus mutabilis Sweet)
The Andean lupine, locally known as ‘tarwi’ or ‘chocho’ (Lupinus mutabilis Sweet) has been cultivated, processed and consumed for at least 1500 years, whose genetic variability has adapted to many microclimates [1]. Even before the Spanish conquest did this crop play an important role in high Andean production systems and in feeding the indigenous population [2]. Among legumes, the lupine is characterised by its high-quality protein content, suitability for environmentally robust production, and potential health benefits [3]. In the countries of the Andean region, the annual per capita consumption varies, e.g., in Ecuador, it is 4 to 8kg person-1, much higher than in Bolivia (0.2kg person-1) and Peru (0.5kg person-1). However, for the year 2017, production did not meet domestic demand in Ecuador, reporting a deficit of approx. 6,000 tons [4]. The gastronomic versatility and nutritional qualities of this legume crop, combined with the work carried out for more than 20 years by both public and private entities in technological innovations, post-harvest, added value, quality seed, improved varieties, among other aspects, have renewed interest in this cultivation [5-7].
Introduction (Description, Domestication/Cultivation History, Including Current Cultivation, Geographical Distribution, Uses)
Introduction
The history of this species as an Andean subsistence crop demonstrates its potential as a crop for low-input agriculture in temperate climates [8]. The selection activities of Andean farmers have represented the only means of domestication of the lupine, giving rise to semidomesticated forms characterized by indehiscent legumes/pods, large seeds, multicolored flowers, highly branched architecture and a more or less annual life cycle [9]. It is a robust crop that can be grown in poor soils and dry climates [1], which stands out for its great potential in soil recovery due to its ability to fix nitrogen [10]. Furthermore, in addition to its high alkaloid content (4.5g 100g-1 dw) [11], the crop has a high resistance to microbial infections and insect attacks [12]. The presence of these alkaloids in the seeds and the low yields (800-1,300kg ha-1) have strongly limited the expansion of this crop [13,14]. Efforts have been made to reestablish lupine as a crop in South America and to adapt it to conditions in Europe [15].
The Andean lupine is characterized by the highest grain quality of all cultivated lupines, presenting an oil content similar to that of soybeans (Glycine max (L.) Merr.) [16]. Numerous studies investigating the nutritional profile and potential applications of this pulse have found a wide range of possible products ranging from proteins, oils and food additives to cosmetics, medicines and biopesticides. Also, the nutritional advantages make it an ideal product for the transition from meat-intensive diets to diets based on vegetable proteins [16].
Origin, diversification and domestication
The oldest evidence of cultivated Andean lupine is related to the seeds found in tombs of the Nazca culture and representations in Tiahuanaco ceramics in Peru [17,18]. The oldest archaeological evidence of domesticated L. mutabilis seeds has been found in the Mantaro Valley in central Peru and dates back to approx. 1800BP. The use of RADseq in the analysis of this archaeological material confirms that L. mutabilis was first domesticated in the Cajamarca region (northern Peru), from the wild progenitor L. piurensis C.P.Sm. Demographic analysis suggests that L. mutabilis separated from its parent around 2600BC and suffered a bottleneck in domestication, with subsequent rapid population expansion as it was cultivated in the Andes [19]. Lupinus mutabilis is reported in Eastern South America, from Colombia to northern Argentina, and with a wide altitudinal range from 1500 to 3800m a.s.l. [20].
In the Andean region 83 species have been identified; the wild relatives that show diversity and variability found in the Andean lupine are the following species: Lupinus ananeanus Ulbr., L. aridulus C.P.Sm., L. ballianaus C.P.Sm., L. chlorolepis C.P.Sm., L. condensiflorus C.P.Sm., L. cuzcensis C.P.Sm., L. dorae C.P.Sm., L. eriocladus Ulbr., L. gibertianus C.P.Sm., L. macbrideianus C.P.Sm., L. microphyllus Desr., L. paniculatus Desr., L. sufferuginous Rusby, L. tarapacencis C.P.Sm. and L. tomentosus DC. [21].
Germplasm collections
The germplasm collections were started in 1974 by Dr Oscar Blanco at the University of Cusco (Peru) and soon spread to Bolivia and Ecuador; Currently, South American institutions have more than 3,000 Andean lupine genotypes. The largest and most relevant germplasm collections of L. mutabilis are found in the gene banks of Peru, Ecuador and Bolivia. However, there are also smaller collections in Chile, Argentina, Colombia, Australia, Russia, Poland, Germany, Spain, Hungary, the United Kingdom and Portugal [20]. Regarding the Ecuadorian collection of Lupinus, the National Institute for Agricultural Research (INIAP) has a collection of approx. 530 accessions of which about 70% belong to L. mutabilis Sweet (Table 1).
Table 1: Number of accessions of L. mutabilis conserved in the INIAP germplasm bank.

Note: Germplasm collected: *period 1975-1999; ** period 2014-2015.
Botanical description
Three geographically separated morpho-types of the Andean
lupine have been suggested based on the considerable genetic
and morphological variability and wide ecological adaptation in
the Andean zone: a) Lupinus mutabilis, lupine (northern Peru and
Ecuador), of more prolific branching, very late, greater hairiness
of leaves and stems, some ecotypes behave as a biennial, tolerant
to anthracnose; b) Lupinus mutabilis, tarwi (central and southern
Peru), scarcely branched, moderately late, somewhat tolerant to
anthracnose; and c) Lupinus mutabilis, tauri (highlands of Peru
and Bolivia), smaller (1-1.40m) with a developed main stem, very
early, susceptible to anthracnose [13,22,23]. High diversity seed
characteristics include shape (lenticulate to spherical), primary
and secondary seed colour, as well as distribution patterns; colour
can range from pearly white to solid black and includes beige/
yellow, brown, dark brown, and colors in between such as brownish
green and greyish green. Most of the seeds have a secondary colour
distribution in darker shades of the primary colour; the secondary
colour distribution also varies among a wide range of patterns,
such as brow-shaped, crescent, mottled or spotted, which can be
expressed either solitary or in combination [13,24].
The presence of considerable variation in germplasm is shown
by different phenotypic traits, such as a wide range of growth
periods, branching patterns, colour and shape of grains and flowers,
as well as flowering times. Both the Inter-Simple Sequence Repeat
(ISSR) and Single- Sequenced Repeat (SSR) markers have revealed
a broad genetic diversity among L. mutabilis lines [25,26], which
could be related to the mixed pollination system that the species
has, and which could explain the presence of the test colour [25].
Environments where lupine is grown
The requirement for lupine is variable, depending on the soil, temperature and wind. It grows well in temperatures from 20 to 25 oC; grain development is optimal below 9.5 oC (night temperature), a condition that occurs in the high Andean region [23]; the early ecotypes of Puno-Peru, needs 450mm of precipitation per crop cycle, while the late ecotypes require between 600 and 700mm [13]. The lupine prefers sandy loam soils, with a thick, deep texture, with a balance of nutrients, good drainage with a pH of 5 to 7 [21,27,28]. In the seedling stage it is susceptible to frost (-4 °C), the higher the temperature, the greater the growth and development, on the contrary, at less than zero degrees Celsius, development and evapotranspiration are inhibited [18].
Uses
Chocho can be consumed directly as a snack [29] and as an ingredient in different products such as fresh salads, soups, cakes, cookies, bread, hamburgers, baby food [29-32]. The new uses of lupine are related to the extraction of oil and production of vegetable milk, yoghurt, obtaining flours and by-products for animal feed [33,34]. Alkaloids such as lupine and sparteine present in the leaves, stem and seed of lupine plants were traditionally used, in combination with paico (Dysphania ambrosioides (L.)) Mosyakin & Clemants, syn. Chenopodium ambrosioides L., a wild relative of quinoa) to repel pests on potato crops such as the Andes weevil (Premnotrypes spp.) and kona kona (Eurysacca quinoae P.) the primary pest of quinoa. In livestock, it is used to control internal and external parasites, practices that are disappearing due to the promotion of industrial agrochemicals [35]. Lupine seeds are used for consumption after debittering [30,36] reducing the alkaloid content to 0.02% for people and from 0.4 to 0.6% for pigs, ruminants and poultry [18,37]. To eliminate antinutritive substances (alkaloids), a hydro-thermal process is carried out, which consists of hydrating the dry grain and soaking it for 12 to 14 hours, then it is cooked for 30 to 40 minutes. The seed is left in a stream of continuous drinking water for three or four days, or in circulating water from the river or streams between seven and ten days [38].
Assets/Benefits (Nutrition, Growing Practices, e.g., Crop Rotation, etc.)
Current situation of lupine in Ecuador
The Andean lupine is currently of agricultural importance only in Ecuador, Peru and Bolivia [39]. The area sown according to the III National Agricultural Census was 5974ha, with an average yield of 400kg ha-1, however, with the introduction of improved varieties, the yield oscillates between 1500kg ha-1 [4,40]. Data from [41] report a sown area of 3,642ha, with a production of 1,339 tons and a yield of 3,678kg ha-1. The lupine is produced in the altitudinal strip that goes from 2500m a.s.l., parallel to the cereal area, up to 3400 or 3600m a.s.l. with risks of frost and hailstorms [37]; the provinces where production is centred are Cotopaxi, Chimborazo and Pichincha [40]. Ecuador has two improved varieties of Andean lupine: INIAP 450 ‘Andino’ and INIAP 541 ‘Guaranguito’ from Peruvian lines that have a short crop cycle (6 months) and a high alkaloid content [42].
Crop husbandry
In the highlands of Colombia, Ecuador, Peru, Bolivia and Chile, the Andean lupine and its wild relatives (kela or kera) constitute one of the components of agroecosystems and ecosystems. The consumption of Andean lupine decreased notably since colonial times because it was replaced by the introduced broad bean (Vicia faba L.) in the crop rotation system [35]. Studies have demonstrated that the Andean lupine can incorporate between 200 and 500kg nitrogen ha-1 into the soil; i.e., an amount equivalent to 350-750kg of urea ha-1 [43]. This is due to the photosynthetic efficiency in converting atmospheric carbon into structural carbon (similar to C4 crops), with its ability to fix atmospheric nitrogen in symbiosis with different species of bacteria and with its ability to solubilise phosphorus from the soil [35,44].
Harvest residues are used as green manure, and the dried stems as fuel due to their large amount of cellulose that provides an excellent calorific value [45]. Something particular that happens in Andean ecosystems is that after earthworks, the first thing that emerges and covers the soil in the plots at rest is a diversity of wild relatives of the Andean lupine, which contributes to the recovery of soil fertility [35].
Nutritional aspects
The Andean lupine, compared to other legumes such as soybeans and beans (Phaseolus vulgaris L.), has enormous nutritional and nutritional potential. The protein content ranges between 40 and 51%, with a high globulin and albumin content, but it is low in tryptophan; it has calcium, iron, zinc, and a high oil content (18 to 22%), in which fatty acids such as linolenic and linoleic predominate (Table 2) [34].
Linoleic acid has properties, which in human metabolism are unique and irreplaceable during specific stages such as pregnancy and the first months of postpartum life. Also, it increases defences and lowers blood pressure, while oleic acid reduces the risk of cardiovascular disease and is antitumor [46]. It prevents chronic diseases such as diabetes, gout, kidney problems, diuretic and emollient [47], it also has antioxidant properties due to the presence of isoflavones [25].
Table 2: Average of the nutritional and functional components of lupine.
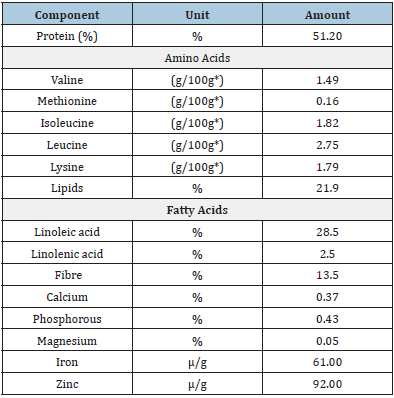
Note: *Content per 100g fresh sample.
Source: [34].
Limitations: Reproduction Needs
From an agronomic point of view
i. One limitation is the lack of early maturing and high-yielding
genotypes, the lack of locally adapted genotypes [15], and the
lack of good quality seed production [39].
ii. Limited research in plant breeding: recent domestication
and history of reproduction fragmented in time and space
have also contributed to the lack of genetic improvement and
lower yields; lack of participatory approaches with farmers for
the selection of local ecotypes. Besides, the lack of advanced
biotechnological methods in genetics, molecular cytogenetics
or tissue culture, has limited the possibility of exploiting natural
variability and performing distant crosses and haploidisation
of material from reproduction [16].
iii. Presence of phytophagous insects: in Ecuador, the increase
in demand intensified the ancestral production system of
Andean lupine, with the use of improved varieties and broader
cultivated areas, which caused the presence of these insects
and the indiscriminate use of insecticides [48].
From a nutritional point of view
i. The Andean lupine has a bitter taste as a result of the high
content of alkaloids, which limits direct consumption for both
human and animal consumption [49]. Hence, it is necessary to
improve the debittering processes [39,50].
ii. Lack of use since the colonial and the republican times: these
crops were devalued, minimizing their consumption and even
disappearing because they were considered “Indian food”
or “poor man’s food” [5]. The challenge is to recognise that
Andean grain and pulse crops contribute to food sovereignty
[39].
iii. Generate specific data about the consumption of the Andean
lupine or tarwi, which will be very useful for making decisions
and actions that allow the promotion of greater consumption
[39].
Future Potential
i. Future work should aim at the development of bitter/sweet lines, with a sufficient level of alkaloids in vegetative tissues to decrease the presence of pathogens [51]. Also, integrated pest and disease management programs are required to improve farmers’ production systems [49].
ii. The combined use of germplasm and modern approaches to broaden the genetic base could help introgression of desirable adaptive traits for environments adapted to certain latitudes [16].
iii. Converting production into a dual-purpose alternative (protein and oil) similar to soybean could be an economical alternative for the productive and competitive development of the Andean region [3].
iv. Andean lupine alkaloids could be of commercial importance due to their pharmacological activity [12,52]. Furthermore, specific protein isolates and concentrates could be of commercial importance due to their functional properties for the chemical and food industry [11,53-55]. Furthermore, the presence of ferritine (protein-rich in Fe) in the protein profile [56] increases the nutritional value of this culture by offering a safe way to increase the intake of iron in the diet [57].
v. In the medical field, quinolizidine alkaloids (Qas) also have an essential role due to multiple properties such as antiarrhythmic, anti-inflammatory, diuretic and hypotensive effects among others [58]. Besides, QAs can also find application in agriculture as a bio-stimulant increasing the growth and yield of other crops [59], as antibacterial agents [60] or as biocidal agents that replace synthetic toxins [61].
References
- Haq N (1993) Underutilized crops. In pulses and vegetables. Chapman & Hall, London, U.K.
- FAO (2016) Legume consumption and production has lost strength in latin america and the caribbean compared to more commercial crops.
- Lucas MM, Stoddard F, Annicchiarico P, Frias J, Martinez-Villaluenga C, et al. (2015) The future of lupin as a protein crop in Europe. Front Plant Sci 6: 1-6.
- Mazón N (2018) Chocho or tarwi as a genetic resource in the Andean region [Online seminar]. Quito, Ecuador: Inter-learning-IPDRS.
- Horton D (2014) Collaborative research on Andean grains in Ecuador. McKnight Foundation and National Institute for Agricultural Research. Quito, Ecuador, pp. 1-43.
- Nájera S (2015) Does lupine ( mutabilis) production have the potential to increase agricultural income and contribute to Ecuador's food security if it replaces soybean or corn production in Ecuador? School of Geociences. University of Edinburgh. Pp. 1-78.
- Márquez C (2016) Planting lupine is more profitable. Leaders Magazine.
- Cowling WA, Buirchell BJ, Tapia ME (1998) Promoting the conservation and use of underutilized and neglected crops. International Plant Genetic Resources Institute, Rome, Italy, Pp. 1-105.
- Clements JC, Sweetingham MS, Smith L, Francis G, Thomas G, et al. (2008) Crop improvement in Lupinus mutabilis for Australian agriculture-progress and prospects. Lupins for health and wealth. International Lupin Association 1: 244-250.
- De Ron A, Sparvoli F, Pueyo J, Bazile D (2017) Protein crops: Food and feed for the future. Front. Plant Sci 8: 1-4.
- Gueguen J, Cerletti P (1994) Proteins of some legume seeds: Soybean, pea, fababean and lupin. New and Developing Sources of Food Proteins pp. 145-193.
- Ciesiolka D, Gulewicz P, Martínez-Villaluenga C, Pilarski R, Bednarczyk M, et al. (2005) Products and biopreparations from alkaloid-rich lupin in animal nutrition and ecological agriculture. Folia biologica (Kraków) 53: 59-66.
- Tapia ME (2015) El tarwi, lupino Andino. Tarwi, tauri o chocho pp. 1-108.
- Galek R, Sawicka-Sienkiewicz E, Zalewski D, Stawiński S, Spychała K (2017) Searching for low alkaloid forms in the Andean lupin (Lupinus mutabilis) collection. Czech J Genet Plant Breed 53(2): 55-62.
- Caligari P, Römer P, Rahim M, Huyghe C, Neves-Martins J, et al. (2000) The potential of Lupinus mutabilis as a crop. in linking research and marketing opportunities for pulses in the 21st Proceedings of the Third International Food Legumes Research Conference pp. 569-573.
- Gulisano A, Alves S, Martins J, Trindade L (2019) Genetics and breeding of Lupinus mutabilis: An emerging protein crop. Front Plant Sci 10: 1385.
- FAO (1992) Marginalized crops, another perspective from 1492. pp. 1-345.
- Tapia M, Fries A (2007) Field guide to andean crops. FAO, Perú.
- Atchison GW, Nevado B, Eastwood RJ, Contreras-Ortiz N, Reynel C, et al. (2016) Lost crops of the Incas: Origins of domestication of the Andean pulse crop Tarwi, Lupinus mutabilis. American Journal of Botany 103(9): 1592-1606.
- Jacobsen S, Mujica A (2008) Geographical distribution of the andean lupin (Lupinus mutabilis Sweet). Plant Genetic Resources Newsletter 155: 1-8.
- Jacobsen S, Mujica A (2006) The tarwi (Lupinus mutabilis Sweet) and its wild relatives. Economic Botany of the Central Andes pp. 458-482.
- Tapia M, Mujica S, Canahua A (1980) Origin, geographical distribution and production systems of quinoa. AGRIS, Peru.
- Gross R (1982) The cultivation and use of Lupinus mutabilis FAO, Rome.
- Falconí C (2012) Lupinus mutabilis in ecuador with special emphasis on anthracnose resistance. Wageningen University Pp. 1-150.
- Chirinos-Arias M, Jiménez E, Vilca-Machaca S (2015) Analysis of Genetic Variability among thirty accessions of Andean Lupin (Lupinus mutabilis Sweet) using ISSR molecular markers. Scientia Agropecuaria 6(1): 17-30.
- Galek R, Sawicka E, Zalewski D (2007) Evaluation of interspecific hybrids of Andean lupin and their parental forms with regard to some morphological and quantitative characters. Fragmenta Agronomica 24(94): 81-87.
- Meneses R (1996) Legumes in Bolivian Agriculture. Rhizobiology Bolivia Project pp. 209-225.
- FAO (2000) Under-exploited Andean crops and their contribution to food. AGRIS pp. 205.
- Villacrés E, Peralta E, Alvarez M (2003) Pussies at their point. AGRIS pp. 1-43.
- Cremer H (1983) Current aspects of legumes as a food constituent in Latin America with special emphasis on lupines: Introduction. Plant Foods for Human Nutrition 32: 95-100.
- Ruales J, Pólit P, Nair B (1988) Nutritional quality of blended foods of rice, soy and lupins, processed by extrusion. Food Chemistry 29(4): 309-321.
- Güémes N, Peña R, Jiménez C, Dávila G, Calderón G (2008) Effective detoxification and decoloration of Lupinus mutabilis seed derivatives, and effect of these derivatives on bread quality and acceptance. Journal of the Science of Food and Agriculture 88(7): 1135-1143.
- Tapia M (1982) Agro-industrial process of tarwi (Lupinus mutabilis). In Proceedings of the International Lupinu Conference. International Lupinu Association. Torremolinos, Spain.
- Peralta E, Villacrés E (2015) 100 practical recipes using quinoa, lupine and amaranth. Miscellaneous publication, Ecuador.
- Canahua A, Román P (2016) Tarwi Andean legume with great industrial potential. LEISA 32(2): 20-22.
- Villacrés E, Caicedo C, Peralta E (2000) Potential zoning, production systems and artisanal processing of lupine (Lupinus mutabilis Sweet) in Ecuador. Quito, Ecuador: INIAP, Santa Catalina Experimental Station. National Legume Program pp. 24-41.
- Caicedo C, Peralta E (2000) Potential zoning, production systems and artisanal processing of lupine (Lupinus mutabilis Sweet) in Ecuador. Quito, Ecuador: INIAP, Santa Catalina Experimental Station. National Legume Program pp. 134.
- Caicedo C, Peralta E, Villacrés E, Rivera M (2001) Postharvest and market of lupine (Lupinus mutabilis Sweet) in Ecuador. Quito-Ecuador.
- Mercado G, Davalos J, Hivos C (2018) Memory virtual forum: The roads of the tarwi and Andean integration: Bolivia, Peru and Ecuador. Bolivia: IPDRS.
- MAGAP (2014) Economic agroecological zoning of the lupine crop (Lupinus mutabilis) in Ecuador at a scale of 1: 250,000. Quito, Ecuador.
- FAOSTAT (2019) Lupine production data in Ecuador.
- Caicedo C, Peralta E, Murillo A, Rivera M, Pinzón J (2010) INIAP 450 Andean. Lupine variety for the Ecuadorian Sierra. Informative bulletin Quito, Ecuador.
- Murillo A, Román P (2016) Andean legume with great potential. Leisa Journal of Agroecology 32(2): 20-21.
- Tay U (2009) Production of canola, lupine and pea in the foothills of Bio and the dry coastal area of the province of Arauco. Institute of Agricultural Research pp. 166-188.
- Basantes E (2015) Management of Andean crops in Ecuador.
- Carrillo C, Cavia M, Alonso-Torre S (2012) Antitumor effect of oleic acid; mechanisms of action: A review. Hospital Nutrition 27(6): 1860-1865.
- Pochettino M, Arenas P, Hurrell J (2011) Plants "to eat and heal." Museum Magazine 25: 48-55.
- Mina D, Struelens Q, Barragán A, Dangles O (2018) The cunt. A superfood that could become a threat. Our Science Magazine 20: 19-21.
- Guerrero M (1987) Some properties and applications of lupine alkaloids (Lupinus mutabilis Sweet). Information and dissemination event of research results on lupine and training in new laboratory techniques. Technical University of Ambato.
- Peralta E (2010) Production and distribution of good quality seed with small Andean grain farmers: Lupine, quinoa, amaranth. National Autonomous Institute of Agricultural Research (INIAP), Quito-Ecuador.
- Wink M (1990) Plant breeding: Low or high alkaloid content. In Proceedings of the 6th International Lupin Conference pp. 326-334.
- Jiménez-Martínez C, Hernandez H, Dávila-Ortíz G (2003) Lupines: An alternative for debittering and utilization in foods. (1st edn) Food Science and Food Biotechnology pp. 233-252.
- Sathe SK, Deshpande S, Salunkhe D (1981) Functional properties of lupin seeds (Lupinus mutabilis) proteins and protein concentrates. Journal of Food Science 47(2): 491-497.
- Doxastakis G (2000) Lupin seed proteins. Developments in Food Science 41: 7-38.
- Moure A, Sineiro J, Domínguez H, Parajó J (2006) Functionality of oilseed protein products: A review. Food Research International 39(9): 945-963.
- Strozycki P, Szczurek A, Lotocka B, Figlerowicz M, Legocki A (2007) Ferritins and nodulation in Lupinus luteus: iron management in indeterminate type nodules. Journal of Experimental Botany 58(12): 3145-3153.
- Zielinska M (2015) Plant ferritin-A source of iron to prevent its deficiency. Nutrients 7(2): 1184-1201.
- Bunsupa S, Katayama K, Ikeura E, Oikawa A, Toyooka K, et al. (2012) Lysine decarboxylase catalyzes the first step of quinolizidine alkaloid biosynthesis and coevolved with alkaloid production in Leguminosae. Plant Cell 24(3): 1202-1216.
- Przybylak J, Ciesiolka D, Wysocka W, Garcia-López M, Ruiz M, et al. (2005) Alkaloid profiles of Mexican wild lupin and an effect of alkaloid preparation from Lupinus exaltatus seeds on growth and yield of paprika (Capsicum annuum L.). Industrial Crops and Products 21(1): 1-7.
- Romeo FV, Fabroni S, Ballistreri G, Muccilli S, Spina A, et al. (2018) Characterization and antimicrobial activity of alkaloid extracts from seeds of different genotypes of Lupinus spp. Sustainability 10(3): 1-12.
- Bermúdez K, Herrera J, Brito R, Wink M, Legal L (2009) Activity of quinolizidine alkaloids from three Mexican Lupinus against the lepidopteran crop pest Spodoptera frugiperda. BioControl 54: 459-466.
© 2021 Marten Sørensen. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






