- Submissions

Full Text
Biodiversity Online J
Thermo-Mineral Springs, Old and Unique Aquatic Ecosystems to Survey and Preserve
Aude Beauger1,2*, Lory-Anne Baker2,3, Carlos E Wetzel4, Luc Ector4 and David Biron2,3
1GEOLAB, Clermont Auvergne University, France
2LTSER “Zone Atelier Uraniferous Territories”, France
3Laboratory “Microorganisms: Genome and Environment” (LMGE), University Clermont- Auvergne, France
4Luxembourg Institute of Science and Technology (LIST), Environmental Research and Innovation, Luxembourg
*Corresponding author: Aude Beauger, GEOLAB, Clermont Auvergne University, CNRS, France
Submission: March 15, 2021; Published: June 11, 2021

ISSN 2637-7082Volume1 Issue4
Abstract
Thermo-mineral springs are particular ecosystems that are present all over the world. However, they are largely disregarded by monitoring programs. They present a wide variety of physical and chemical properties of waters leading to a wide spectrum of interesting habitats available for the development of diatoms, such as new species recently described, that could be considered as endemic for some of them. During a survey performed in the French Massif Central, diatoms were sampled in different springs. Some of these emergences are natural, while others are Man-made springs. Significant differences are observed between species richness of these two types of springs. The natural springs tend to present higher richness than Man-made ones. This highlight the importance of restoring thermo-mineral springs and to suppress constructions done around the emergence when use is not on-going anymore. This would allows the reestablishment of these aquatic habits, favouring the development of unique diatom species.
Keywords: Thermo-mineral springs; Diatoms; Species richness; Restoration; Protection
Introduction
Thermo-Mineral Springs (TMS) are particular ecosystems that are present all over the world. However, springs are largely disregarded by monitoring programs. For instance, they are not explicitly considered by the environmental policy and legislation of the European community (Water Framework Directive, 2000/60/CE) [1] promulgated to prevent further deterioration, and to protect and enhance the status of aquatic ecosystems [2,3]. In this European directive, freshwater environments such as rivers and lakes are mainly considered. Concerning Habitats-Fauna-Flora directive (92/43/CEE) [4] there is only a reference to mineral springs about the development of acid sphagnum peatlands and to Cratoneurion related to limestone marshes. Thus, TMS are not taken into account by European commission and the European environment agency to achieve ‘‘good ecological status’’ in these particular aquatic ecosystems, and then to protect and restore these ones. In fact, only the IUCN Red List of Ecosystems Categories and Criteria could be used to assess the ecological status of TMS [5].
TMS are studied by diatomists all over the world and particularly in Europe [6-12]. These
studies highlighted a wide variety of physical and chemical properties of waters leading to
a wide spectrum of habitats available for the development of diatoms. Moreover, during the
different survey done on TMS, new taxa are regularly described [8-10,13-19]. The presence
of these undescribed and listed species that could be considered as endemic for some of
them, highlights the importance of protecting these extreme and particular habitats of the
biosphere. Indeed, springs can be considered as isolated aquatic ecosystems whose conditions have changed very little over the past millenia, thus providing in
some cases reference conditions and an exceptional window into
the history of life on earth [20]. Moreover, as the observations
done in Poland, the TMS offer a reservoir of threatened species [8].
Currently, linked to insufficient protection of diatoms by regional,
national, European and global legislation, the destruction of their
natural habitats is the main result. This is the case for many springs
of the French Massif Central (FMC) located at the southern part of
France.
The FMC is the largest massif of France (>85,000km2). Due to
its surface area, this region, the Auvergne, includes one-third of the
French TMS. In 1864, Lecoq [21] recorded more than 450 mineral
springs, such as the most radioactive of France [22]. In the FMC,
few of the springs have kept their original form (i.e., natural spring)
because they have been used by man for a long time. Indeed, some
of TMS were used since the Gallo-Roman period for therapeutic
purposes. Moreover, bottled water industry uses many springs: for
example, the mineral water St. Yorre near Vichy but also some ones
are collected for artisanal activities (creation of petrified objects).
An on-going investigation made on springs of the FMC (not
used for any type of activities) reveals that these habitats shelter
hot-spots for biodiversity [10,17,18,23]. Species such as Navicula
sanctamargaritae Beauger [10] are widely abundant in high
conductivity (ranging between 2,000 and 10,000μS.cm-1) (Beauger,
personal communication) while Sellaphora labernardierei Ector
prefers the presence of nitrates (around 10mg L-1) [17]. All
these characteristics and observations could be useful tools for
monitoring and evaluation of the ecological status of springs as
diatoms are classically used for bio-assessment. This inventory also
underlined that 10% of the springs have disappeared since 1864,
either destroyed due to human activity or to their abandonment.
Moreover, the layout of springs induces an artificialization and
anthropization of the habitat, i.e., the conversion of natural
environments by human action. All the human activities lead to
the degradation of the biodiversity of TMS [24] or could induced
a disappearance of species. Nevertheless, biodiversity is the
keystone of the functioning of these ecosystems from which derive
ecosystemic services useful for human societies.
The project DISCOVER started in 2019 focused on the study of
diatoms communities inhabiting 22 mineral springs of the FMC: 1)
12 TMS emerged in a naturally context; 2) for 10 others, springs are
characterised as Man-made springs or springs with medium-to-high
anthropogenic activities (Figure 1, Annex 1). The mean of diatom
species number in natural springs was 15±3 in autumn 2019 and
16±7 in spring 2020. Man-made springs showed generally lower
richness with a mean of 7±5 for autumn 2019 and spring 2020. A
Kruskal-Wallis tests reveals significant difference between species
richness of natural and Man-made springs (p=0.003 in 2019 and
p=0.001 in 2020) (Figure 2) and no difference when considering
the Shannon index.
Figure 1: Man-made spring a. (named “Salins”) vs natural spring b. (named “Salut”).

Annex 1: Data on thermo-mineral springs configuration and the number of taxa observed in Autumn 2019 and Spring 2020: MM= Man-made springs; N= Natural springs.
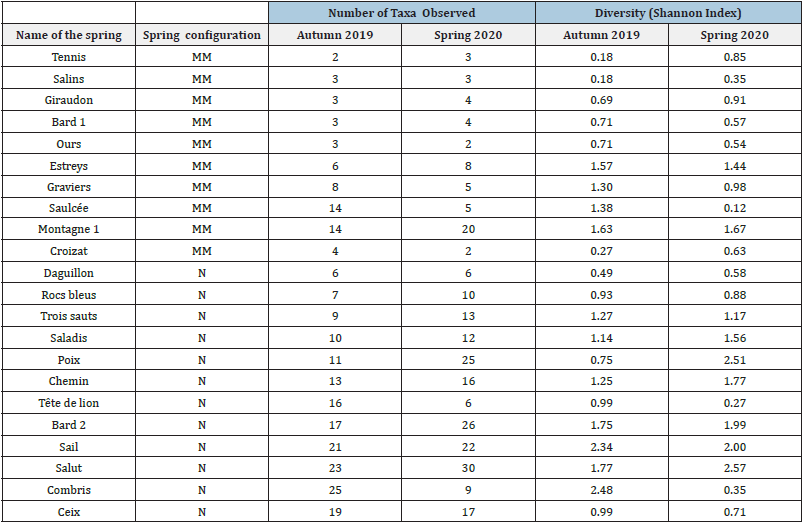
Figure 2: Kruskal-Wallis tests on richness of different mineral springs sampled in
A. Autumn 2019 and
B. Spring 2020. N: Natural springs (n=12); MM: Man-made springs or springs with medium-to-high anthropogenic activities (n=10). *: significant p-value with α level = 0.05.
This study underlines the importance of restoring the thermo-mineral springs and to suppress the construction made around the emergence when use is stopped. This would permit the prompt reestablishment of these aquatic habits and favour the establishment of unique diatom species. Not only is diatom biodiversity increasing, but the reappearance and development of plants typical of such habitats such as Triglochin maritima also happens. This improvement enhances consequently the ecological status of the thermo-mineral springs and the ecosystemic services offer by this aquatic habitat.
Acknowledgement
This study was funded by a grant from the National Center for Scientific Research (CNRS, PRIME 80 CNRS/NEEDS, project Acronym: DISCOVER) and from Clermont-Metropolis (Acronym project: ORIGIN). We acknowledge financial support from CNRSINEE within the context of the Uranium Territories Workshop Zone.
References
- Segadelli S, Cantonati M, Bertoni E, Spitale D, Angeli N, et al. (2015) Can reference spring diatom communities be predicted from simple aquifer and emergence-site characteristics? Conference: 9th Use of algae for monitoring rivers and comparable habitats and International workshop on benthic algae taxonomy-MUSE, Trento, Italy, pp. 37.
- Logan P, Furse M (2002) Preparing for the european water framework directive making the links between habitat and aquatic biota. Aquatic Conservation: Marine and Freshwater Ecosystems 12(4): 425-437.
- Irvine K (2004) Classifying ecological status under the european water framework directive: The need for monitoring to account for natural variability. Aquatic Conservation: Marine and Freshwater Ecosystems 14(2): 107-112.
- EEC Council (1992) Directive 92/43/EEC of 21 May 1992 concerning the conservation of natural habitats and of wild fauna and flora. Latest modification: Council Directive.
- IUCN (2015) Guidelines for the application of IUCN red list of ecosystems categories and criteria. Switzerland Pp. 93.
- Ector L, Iserentant R (1988) The diatoms of the fontinal groups of the Val de Bagnes (Valais, Switzerland). Memory of the Royal Botanical Society of Belgium 10: 12-16.
- Werum M, Lange-Bertalot H (2004) Diatoms in springs from Central Europe and elsewhere under the influence of hydrogeology and anthropogenic impacts. Iconographia Diatomologica 13: 1-417.
- Żelazna-Wieczorek J (2011) Diatom flora in springs of Łódź Hills (Central Poland). Biodiversity, taxonomy, and temporal changes of epipsammic diatom assemblages in springs affected by human impact. Diatom Monographs 13: 1-419.
- Wojtal AZ (2013) Species composition and distribution of diatom assemblages in spring waters from various geological formations in southern Poland. Bibliotheca Diatomologica 59: 1-436.
- Beauger A, Voldoire O, Mertens A, Le Cohu R, Van de Vijver B (2015) Two new Navicula species (Bacillariophyceae) from Western Europe. Phytotaxa 230(2): 172-182.
- Delgado C, Feio MJ, Gonçalves V, Raposeiro P, Almeida SFP (2019) Thermo-mineral sources in mainland Portugal and Azores islands. University of Coimbra Press, Coimbra, Portugal, pp. 423-441.
- Lai GG, Padedda BM, Wetzel CE, Cantonati M, Sechi N, et al. (2019a) Diatom assemblages from different substrates of the Casteldoria thermo-mineral spring (Northern Sardinia, Italy). Botany Letters 166(1): 14-31.
- Reichardt E (2006) Remarkable diatom finds from Bavaria V-New and rare species from the Schwarzachlkamm. Reports of the Bavarian Botanical Society 76: 41-51.
- Cantonati M, Angeli N, Spitale D, Lange-Bertalot H (2016) A new Navicula (Bacillariophyta) species from low-elevation carbonate springs affected by anthropogenic disturbance. Fottea 16(2): 255-265.
- Wojtal AZ (2009) Nupela marvanii nov., and N. lapidosa (Krasske) Lange-Bertalot in Poland with notes on the distribution and ecology of the genus Nupela (Bacillariophyta). Fottea 9(2): 233-242.
- Delgado C, Ector L, Novais MH, Blanco S, Hoffmann L, et al. (2013) Epilithic diatoms of springs and spring-fed streams in Majorca Island (Spain) with the description of a new diatom species Cymbopleura margalefii nov. Fottea 13(2): 87-104.
- Beauger A, Wetzel CE, Voldoire O, Garreau A, Ector L (2016) Sellaphora labernardierei (Sellaphoraceae, Bacillariophyta), a new epilithic species from French spring and four new combinations within the genus Sellaphora. Phytotaxa 260(3): 235-246.
- Beauger A, Wetzel CE, Voldoire O, Ector L (2019) Pseudostaurosira bardii (Fragilariaceae, Bacillariophyta), a new species from a saline hydrothermal spring of the Massif Central (France). Botany Letters 166(1): 3-13.
- Lai GG, Ector L, Lugliè A, Sechi N, Wetzel CE (2018) Sellaphora gologonica nov. (Bacillariophyta, Sellaphoraceae), a new diatom species from a Mediterranean karst spring (Sardinia, Italy). Phytotaxa 356(2): 145-157.
- Cantonati M, Füreder L, Gerecke R, Jüttner I, Cox EJ (2012) Crenic habitats, hotspots for freshwater biodiversity conservation: toward an understanding of their ecology. Freshwater Science 31(2): 463-480.
- Lecoq H (1864) Mineral waters considered in their relation to chemistry and geology. Paris, France, pp. 376.
- Jacquet C (1929) Contribution to the study of the radioactivity of mineral waters and the magnetism of volcanic rocks in the Puy-de-Dôme department. The University Press of France, Paris, France, pp. 130.
- Beauger A, Voldoire O, Wetzel CE, Allain E, Millan F, et al. (2020) Biodiversity and ecology of diatoms in mineral springs of the area of Sainte Marguerite (Saint-Maurice-ès-Allier, Massif central, France). BIOM 1: 21-34.
- Strayer DL, Dudgeon D (2010) Freshwater biodiversity conservation: recent progress and future challenges. Journal of the North American Benthological Society 29(1): 344-358.
© 2021 Giovanni Barozzi Reggiani. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






