- Submissions

Full Text
Biodiversity Online J
Low Germination Success in Phyteuma cordatum Balb and Empetrum hermaphroditum Hagerup
Ivan Pace* and Paola Maria Chiavazza
Department of Agricultural Forest and Food Sciences (DISAFA), University of Turin, Italy
*Corresponding author: Chiavazza Paola Maria, DISAFA, University of Turin, Italy
Submission: August 18, 2020; Published: October 23, 2020

Volume1 Issue2October, 2020
Introduction
This study aimed at carrying out experimental laboratory activities on some spontaneous plant species present within areas of the Piedmontese Natura 2000 Network, with the aim of identifying the regeneration protocol for their ex situ conservation. In Europe and around the world, climate change and human actions are compromising the conservation status of natural habitats, spontaneous plant and animal species. To cope with these changes, the European Union has set up the Natura 2000 Network, a network of sites of community interest (SIC) and special protection areas (SPA) created in the regulatory framework of the “Habitats Directive (92/43/EEC) and the Birds Directive” (79/409/EEC), for the protection and conservation of habitats, animal and plant species identified as priorities by the Member States of the European Union. Conservation methodologies for the protection of biological resources include conservation strategies ex situ that is, out of the natural environment. Micropropagation is a multiplication technique that allows to obtain a clone of the plant itself, or a set of individuals with the same genetic heritage, through the use of in vitro culture methods of cells and plant tissues. These techniques have been used for the propagation of rare and/or endemic species of the Ligurian and Maritime Alps. The study concerned Phyteuma cordatum Balb and Empetrum hermaphroditum Hagerup.
Phyteuma Cordatum
The species is included among the most significant endemics of the Ligurian and French- Italian Maritime Alps [1]. It has a fragmented area in a small number of stations, mostly distant from each other, a clear testimony of its antiquity. Stress-tolerant competitor species [2] occupies habitat 8210 “Limestone rocky walls with chasmophytic vegetation”, on limestone cliffs at altitudes of 1800-2200 meters [3].
Empetrum Hermaphroditum
It is an evergreen shrub with a prostrate and branchy habit, capable of occupying different habitats. Although it presents a circumboreal distribution, in the Ligurian and Maritime Alps it occupies a single station at 2000 meters above sea level and is considered a rare species. Prostrate habit and dense ground cover inhibit seed germination of some competing species, while creating conditions for seed germination and conifer seedling development [4,5].
Materials and Methods
Adult plants of Phyteuma cordatum Balb were collected in their natural environment in an area within the Marguareis Natural Park. Explants about 8/10cm long were collected from few adult plants. To ensure adequate genetic variability and maintain the right humidity, the material taken from some individuals has been stored in plastic bags with little water inside. This material was stored in a refrigerator for a few days before being used in laboratory. Here we proceeded with the preparation of leaves and small explants 1-2 centimeters long, each containing a vegetative apex (Figure 1). These explants were placed in containers for washing in cold running water (10min). Subsequently, sterilization operations were carried out forthe in vitro culture. Sodium hypochlorite was tested by varying time between 30’ and 1h.
Figure 1: Stages of leaves and explants preparation in Phyteuma cordatum Balb plants (I. Pace, 2019).

a. 30min 0.5% NaOCl+Tween 20
b. 60min 0.5% NaOCl+Tween 20
After the sterilization treatments, several rinses were carried out with sterile distilled water to completely eliminate both the surfactant (Tween 20) and the sterilizing agent. MS was used as culture medium, in presence of 2.4D plant growth regulator at different concentrations:
a. MS+2.4D 0.5mg/L
b. MS+2.4D 1mg/L
c. MS+2.4D 2mg/L
The explants were placed in falcon tubes containing culture medium and plant growth regulator and placed in the growth chamber with artificial white light (photoperiod of 12 hours light/ dark) at 25°C. A strong contamination was present and, to contain the problem, we tried to use Plant Preservative Mixture (PPM 3ml/l) by adding it to the culture medium, with scarce results. Using MS culture medium, leaf or explant sections showed no responsiveness for callus formation at plant growth regulator concentrations used.
Sterilization Procedures on Seeds of Alpine Species
Seeds of Empetrum hermaphroditum and Phyteuma cordatum were subjected to sterilization tests, which was followed by the vitality test (TTC) to verify that the same did not compromise their germination. The test took place on (Table 1).
Table 1: NaOCl treatment results.

Viability Test on Seeds of Alpine Species
The chemical test TTC (2,3,5-Triphenyl Tetrazolium Chloride), takes its name from the chemical reagent used which, in contact with the oxygen present in the vital cells, converts into a product called “Formazan”, producing a color reaction observable in still vital tissues. The resulting color at the end of the test can be a more or less marked shade of red for healthy and vital tissues, while the dead or damaged parts do not stain [6]. To evaluate the viability of the seeds of our alpine species, tests were carried out with tetrazolium chloride. The seeds were soaked with a 1% v/v TTC solution, incubated in the dark in an oven at 25 ° C for 1 hour. The sample was then checked under the stereoscope. Based on the tissue response to TTC, seeds are divided into several categories:
a. Reactive, if both the embryo and the endosperm are uniformly colored red;
b. Partially reactive, if only the endosperm or only the embryo or both take on a spotted or uniform pink color;
c. Non-reactive, if neither the embryo nor the endosperm stain;
d. underdeveloped, in the case of incompletely formed seeds;
e. contaminated, if they have loose and rotting tissues;
f. compartments, if they are empty.
This method, widely used by Germplasm Banks [7], is extremely effective, rapid (as the results can be observed after a few hours from the application of the reagent) and advantageous (Table 2), as it allows the use of a reduced number of randomly extracted seeds from the batch to be analyzed.
TTC was tested on a sample of the available seeds of Empetrum hermaphroditum and Phyteuma cordatum collected in 2012 and 2018. The ideal conditions for the conservation of orthodox seeds foresee their dehydration and subsequent storage at low temperatures [6]. On the Seed Information Database (SID) of the Royal Botanic Gardens of Kew (UK), the conservation conditions of the two species are indicated as follows:
Table 2: Empty seeds are not included in the final percentage counting*.

a. Empetrum hermaphroditum: Dehydration up to 15% relative humidity (RH) and freezing at -20 °C (orthodox, i.e. able to tolerate dehydration);
b. Phyteuma cordatum: Despite the lack of data available for the species, of 7 taxa belonging to the genus Phyteuma, 71% are orthodox, while 29% are indeterminate. Although the seeds of both species have been stored for years in an air-conditioned chamber at 10°C and RH 30%, they return good percentages of vitality (Figure 2).
Figure 2: Reactive seeds of Empetrum hermaphroditum where both embryo and endosperm are uniformly coloured in red (I. Pace, 2019).
Germination Test on Empetrum Hermaphroditum and Phyteuma Cordatum Seeds
Our aim was to obtain sterile Phyteuma cordatum and Empetrum hermaphroditum seedlings, to be used as aseptic material for subsequent propagation. For Empetrum hermaphroditum we based on an already existing study [8], while for Phyteuma cordatum we had no data available regarding germination. It was chosen to use only agar (test) and agar with the addition of gibberellins (GA), hormones capable of eliminating seed dormancy, at concentrations of 250 and 1000mg/L (Table 3). The material was incubated under different conditions:
a. culture chamber with thermoperiod 25/15 °C and photoperiod 14h light/10h dark;
b. dark culture chamber with thermoperiod 20 °C.
The freshly germinated seedling was transferred in MS+AG medium, with a photoperiod of 14h light/10h dark and T 20 °C (Figure 3).
Table 3: Germination of one seed of Phyteuma cordatum, maintained for 3 months at T 20°C in the dark.
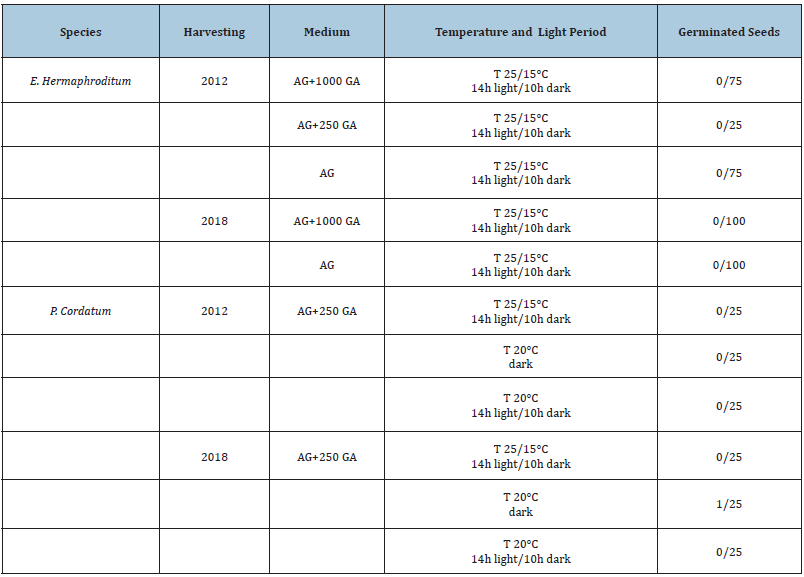
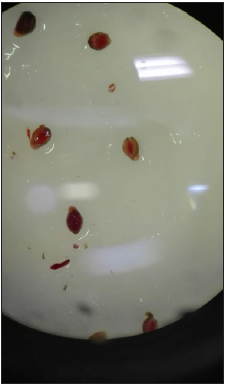
Figure 3: Phyteuma cordatum germinated seed (I. Pace, 2019).

References
- Sana Ak (1990) Honor and shame - women in modern Iraq. Saqi Books, London, UK.
- Andersson A (2003) Honor killings - the survival of patriarchy in different societies.
- Muhammad BG, Prince of Jordan (1999) The tribes of Jordan at the beginning of the twenty-first century.
- Collins PH (1997) Comment on Hekman’s “truth and method: Feminist standpoint theory revisited”: Where’s the power? Signs 22(2): 375-381.
- Collins P (1991) Black feminist thought: Knowledge, consciousness, and politics of empowerment. Routledge, New York, USA, p. 283.
- Nancy H (1983) Money, sex, and power: Toward a feminist historical materialism. Longman, Harlow, United Kingdom, pp. 152-231.
- Hassan Y (1999) The Fate of Pakistani women. International Herald Tribune, New York, USA.
- Amman (2003) Human rights watch interview with Rana Husseini.
- Lober J (1998) Gender inequality: Feminist theories and politics. Roxbury Publishing, Los Angeles, USA.
- Kamala K (2005) Victims and agents of crime: The new crusade against trafficking in Sudbury. Julia, global lockdown race, gender, and the prison - Industrial complex. Routledge, New York, USA.
- Dwight MA (2001) I know what a slave knows Mary prince as witness, or the rhetorical uses of experience in impossible witness truth, abolitionism, and slave testimony. New York University Press, New York, USA.
- Jose EM (2017) Chico, what does it feel like to be a problem? The Transmission of Brownness.
- https://medium.com/the-wvoice/honor-crimes-in-jordan-a5a004f1eb1fm/
- https://medium.com/the-wvoice/honor-crimes-in-jordan-a5a004f1eb1f
- http://hrw.org/arabic/reports/2004/jordan-honor.htm
- Nanes SE (2003) Fighting honor crimes: Evidence of civil society in Jordan. Middle East Journal 57(1): p. 6.
- Franklin S (2000) Jordan begins to punish practice of 'Honor Killings'. The Chicago Tribune, Chicago, USA.
- Amman (2020) Student-designed billing, health apps shine bright at CPF awards. The Jordan Times.
- Reuters (2000) Honor Killings of Women Said. Global Policy Forum.
- http://www.dhushara.com/book/zulu/islamp/jordan/jordan.htm
- http://www.jordanembassyus.org/
- Lynn W, Sara H (2005) Honour crimes, paradigms, and violence against women. Zed Books, London, UK.
- http://www.psd.gov.jo/images/cid/report2016/start.htm
- Wazir AY (2017) Jordan abolishes rape law, it must follow suit with honor killing law.
- https://www.aliftaa.jo/ShowContent.aspx?Id=205
- https://www.khaberni.com/news/
© 2020 Ivan Pace. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






