- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Use of Bacillus Subtilis and Spirulina Platensis in the Diet of Broiler Chickens
Mohammad Aminul Islam1*, Mohammad Fahim Hezaze1 and Masahide Nishibori2
1Department of Dairy and Poultry Science, Bangabandhu Sheikh Mujibur Rahman Agricultural University, Bangladesh
2Lab of Animal Genetics, Graduate School of Integrated Sciences for Life, Hiroshima University, Japan
*Corresponding author: Mohammad Aminul Islam, Department of Dairy and Poultry Science, Bangabandhu Sheikh Mujibur Rahman Agricultural University (BSMRAU), Gazipur-1706, Bangladesh
Submission: November 19, 2023;Published: April 29, 2024

ISSN: 2576-9162 Volume9 Issue4
Abstract
Ninety Cobb-500 straight run broiler chicks were allotted into three dietary groups; D1 (Control diet), D2 (diet with 0.5g Bacillus Subtilis/kg diet), D3 (diet with 10g Spirulina Platensis/kg diet) having 3 replicates in each and 10 chicks/replicate. The birds were reared on a littered floor management system for 35 days of age and fed a starter diet (0-14 days) that contained 22% CP and 2900Kcal ME/kg diet and a finisher diet (15-35 days) contained 19% CP and 3000Kcal ME/kg diet. Dietary groups did not differ statistically for the body weight, feed intake, FCR, mortality, cost of production, and net profit (p˃0.05). However, diet D2 tended to perform the best among the 3 diets in terms of body weight, feed intake, FCR, mortality, production cost, and net profit. Of the two diets, D1 tended to show lower production costs and a higher net profit compared to D3. The highest dressed meat yield and heart weight were noted in D1, followed by D3 and D2, respectively. Hence, diet D2 was comparable to diet D3 in the case of meat yield traits. Diets were found to be similar in total cholesterol, Triglyceride (TG), Low-Density Lipoprotein (LDL), and High-Density Lipoprotein (HDL) (p<0.05). However, D2 tended to be the lowest for total cholesterol, and LDL, and the highest for HDL, followed by D1 and D3, respectively. Therefore, Bacillus Subtilis (0.5gBS/kg diet=D2) may be beneficial for broiler production
Keywords:Broiler chicken; Growth; Lipid profiles; Meat yields; Probiotic; Spirulina
Introduction
Nowadays, broiler meat is very popular with consumers. Because it is easy and cheaper to produce broiler meat within the shortest possible period. Over and above, regardless of age and religion, everybody prefers broiler meat. To rear broiler chicken, different growth promoters and antibiotics are used in the diet of poultry to have maximum quality products, and control disease outbreaks [1]. Using for a long time of antibiotics in poultry feed developed a resistance to drugs [2] and residues [3] in the body of the birds and lowered the beneficial effect of microflora in the gut of the bird [4]. At present poultry industries have moved to reduce in use of antibiotics as medicine because of banding antibiotics in most of the countries to use in poultry rearing. Antibiotics have residual effects on the human body that develop antibiotic-resistant in humans [5]. Therefore, poultry scientists are working to introduce alternatives in poultry farming to have maximum growth without any hazards as well as maximum profit. In this regard, probiotics may be considered to add to the poultry diet. The genus of bacterial like lactobacilli and bifidobacteria are often used as probiotics even though the other beneficial groups of bacteria may be used in the diet that improves the immune system and inhibits the growth of harmful bacteria in the birds [6]. Therefore, the use of prebiotics in the poultry diet may be advantageous to produce quality, safe, and profitable poultry products.
Probiotics are cultures of microorganisms such as yeast and bacteria that have a beneficial effect on the growth and immune system of birds. It has been found in a study that probiotics impacted gut-related lymphoid tissues [7]. Probiotics are very active in developing a mucosal attachment, crucial nutrients, and antimicrobial complexes in the gut of the bird which prevent the growth of pathogenic microbes and their harmful effects [8]. As a result, using probiotics in the diet of bird increase the growth of birds. However, the efficacy of probiotics depends on strains, level of administration, application method, the composition of the diet, bird’s age, survivability in the host, and duration of storage, etc. [9]. In this case, Bacillus Subtilis probiotic is a promising growth promoter in favor of the resistance of its spores in hot-humid climates [10].
A gram-positive bacteria-Bacillus Subtilis can produce endospores resistant to hot environments and secrete enzymes; amylase, protease, and lipase that can degrade plant-based complex carbohydrates [11]. Bacillus can reduce the pH in the gut to maintain the micro ecologic balance in the animals’ intestines and improve animal growth, Feed Conversion Efficiency (FCE), and immune responses [12,13]. It can reduce pH in the intestinal lumina maintain the equilibrium and stability and improve the intestinal microbiota [14]. Therefore, Bacillus is used as a safe and high-quality probiotic or feed additive in the diet of animals or poultry. Latorre et al. [15] suggested using Bacillus Subtilis in a broiler diet for its beneficial effects. As it has a beneficial effect and is non-toxigenic, the European Food Safety Authority (EFSA) proclaimed the safe animal feed additive of B. subtilis for an animal [16]. Bacillus Subtilis improved digestion, growth, and FCR [14,17].
Spirulina Platensis is a Cyanobacterium that is considered a good source of protein, essential Amino Acids, Vitamins; Vitamin B12, Thiamin, Pyridoxine, Vitamin C, Riboflavin, Minerals, β-Carotene, essential Fatty Acids, antioxidants, pigments like carotenoids, and phenolic acids [18]. It has a beneficial effect on arthritis [19], immuno-system, and antiviral effect [20]. Besides, Spirulina has been found to influence immune function, and reproduction and improve the growth of birds. In addition, Ross & Dominy [21] reported superior broiler growth performance on a diet with Spirulina over a control diet. Spirulina promotes nutrient digestion and mineral absorption and protects against diarrhea [22]. Inborr [23] reported that algae meal in diets increased the concentration of carotene in the liver, adipose tissue, and breast muscle. As a result, birds have yellow pigment in their skin compared with the control group. The broilers in the diet with algae meal gained weight faster- utilizing feed significantly. Ross & Dominy [21] showed no adverse effect of dietary Spirulina on body weight and mortality of birds. As per the report of Kaoud [24] and Kharde et al. [25], it was found that birds had improved body weight, carcass yield, and Feed Conversion Efficiency (FCE) in the diet with Spirulina Platensis. Spirulina Platensis is a natural unconventional feed additive that may be beneficial for yielding safe and profitable broilers.
Considering the above facts, this study was aimed at assessing the effect of Bacillus Subtilis and Spirulina Platensis on the performances of growth, meat yield, and blood lipid profiles of broiler chickens to pick out a suitable dietary group for the safe and cost-effective broiler production.
Materials and Methods
Experimental site
The experiment was conducted at Bangabandhu Sheikh Mujibur Rahman Agricultural University (BSMRAU) Livestock and Poultry farm, Gazipur-1706, Bangladesh in 2020-2021.
Collection of Bacillus Subtilis and Spirulina Platensis
Spirulina Platensis commercial name (Eskalina) was collected from a Poultry Medicine shop, Joydebpur, Gazipur, Bangladesh (Figure 1). Bacillus Subtilis probiotic (Figure 2) was collected from Challenge Group, 12 Zhongguancun South Street, Haidian District, Beijing 100081, P.R. China. These two feed items were used to prepare a broiler diet during the investigation.
Figure 1:Spirulina platensis.

Figure 2:Bacillus subtilis.

Preparation of house and diet
Before receiving the chicks, the house was prepared by cleaning and disinfecting correctly and then adjusting the brooding temperature and humidity. The experimental shed was fumigated using formalin and potassium permanganate @2:1 ratio for 12 hours after placing the necessary equipment and utensils to destroy all kinds of microorganisms. After 12 hours of fumigation, the windows of the shed were opened to remove the gas from the house. The 3 diets as mash were prepared using the tested and locally available feed ingredients every week as per the ration required for the broiler chicks (Table 1).
Table 1:Composition of control diet applied in the
experiment.
D1=Control diet (No Spirulina platensis or Bacillus subtilis)
D2=Control diet supplemented with 0.5g Bacillus subtilis/
kg diet
D3=Control diet supplemented with 10g Spirulina
platensis/kg diet
ME=Metabolizable energy.

Feeding trial
A total of 90-1-day-old broiler chicks of Cobb 500 were collected from Nourish Agro Ltd. and randomly assigned into three diets; D1 (control or basal diet), D2 (diet with 0.5g Bacillus Subtilis/ kg diet), D3 (diet with 10g Spirulina Platensis/kg diet) having 3 replications each and 10 chicks/replicate (Table 1). A starter ration or diet contained 22% CP and 2900Kcal ME/kg diet for 0-14 days and a finisher ration or diet contained 19% CP and 3000Kcal ME/ kg diet was offered to the birds for 15-35 days. The experimental birds were brought up on a saw-dust-based littered floor for 35 days of age. Standard management practices were followed during the investigation. No synthetic amino acids, vitamins, minerals, antibiotics, etc. were fed to the birds during the investigation.
Data recording
Following are the growth performance traits recorded during the experimental period: Body weight and feed intake (replicationwise): Every week. Dead birds: when happened. The following traits were calculated using the formula given by Onunkwo & Okoro [26].
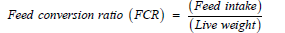
Production cost (Taka/kg live weight): calculated considering the cost of chick, feed, labor, litter, vaccine, etc. Net profit (Taka/kg live weight)=Price (Taka/kg live weight)-Production cost (Taka/kg live weight).
Meat yield traits of broiler chickens fed diets with Bacillus Subtilis and Spirulina Platensis
At 35 days of age, a total of 9 birds from 3 dietary groups had 3 replications each, and 1 bird/replication was taken randomly. The birds were slaughtered, de-feathered, eviscerated, and made cut-up parts to record meat yield traits. The meat yield traits were recorded during the investigation and then calculated as a percentage: Live weight, blood, feather, head, dressed meat, breast meat, dark meat, wings, thigh, drumstick, heart, gizzard, liver, and skin weight. The meat samples of slaughtered bird replication wise were taken and measured dry meat weight using the oven at a temperature of 105 °C for 24hrs.
Lipid profiles of broiler chickens fed diets included Bacillus Subtilis and Spirulina Platensis
The blood samples (10ml/bird) at 35 days of age of the bird during slaughter for recording meat yield were collected replication-wise and then centrifuged at 2400rpm for 15 minutes. The supernatant (blood serum) was transferred into the Eppendorf tube to measure blood lipid profiles of cholesterol, Triglycerides (TG), High-Density Lipoprotein (HDL), and Low-Density Lipoprotein (LDL) in a spectrophotometric method using the cholesterol test kit (Crescent diagnostic cholesterol kit: Cat No. CS 603).
Statistical analysis
The collected data were analysed by ANOVA in a simple Completely Randomized Design (CRD), applying the Statistix10 computer package program.
Statistical model
The following statistical model was followed for data analysis
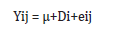
Where Yij is the observation of the jth replication of the ith
dietary groups.
μ is the overall mean.
Di is the fixed effect of the ith dietary groups (i=1, 2, 3).
eij is the random error
Animal welfare and ethical approval
The study was approved by the Institutional Committee on Animal Care and Use in Research (ICACUR) of Bangabandhu Sheikh Mujibur Rahman Agricultural University (No. BSMRAU/DEAN/ FVMAS/25/ICACUR/19).
Result and Discussion
Growth performance of broiler-fed diets with Spirulina Platensis and Bacillus Subtilis
No significant difference was found among the diets for body weight, feed intake, FCR, mortality, production cost, and net profit (p˃0.05) (Table 2). Evidently but not significantly diet D2 performed the best among the diets in terms of the traits; body weight, feed intake, FCR, mortality, production cost, and net profit. Of the two diets, diet D1 had a tendency to show higher net profit than diet D3. Despite the diet was not different statistically, diet D2 tended to be better than Diet D1 or D3 in terms of growth performance which was consistent with Liu et al. [27] and Vimon et al. [28]. They found improved body weight in the diet with Bacillus Subtilis compared to the control diet. In this study, diet D3 had a lower net profit despite having a higher body weight and a lower FCR because of the high price of the Spirulina which affected the production cost as well as net profit. This was supported by Rawshon et al. [29]. They observed the highest body weight and the lowest FCR in the diet containing 8g Spirulina/kg diet. Joya et al. [30] reported the improved body weight of broiler chicken and FCR in diet with individual use of Spirulina Platensis and Bacillus sibtilis.
Table 2:Growth performance of broiler fed diets with Bacillus subtilis and Spirulina platensis at 35 days of age. +D1=Control diet; D2=Control diet supplemented with Bacillus subtilis; D3=Control diet supplemented with Spirulina platensis; NS, P˃0.05.

Meat yield traits (%) of broiler fed the diet with Bacillus Subtilis and Spirulina Platensis
The diet was significantly different for dressed meat yield (p˂0.01) and heart weight (p˂0.05) but not different for live weight, blood, head, shank, breast meat, dark meat, wings, thigh, drumstick, gizzard, liver, skin, and dry meat yield (p˃0.05) (Table 3). The highest dressed meat yield and heart weight were recorded in D1, followed by D3 and D2, respectively. The breast meat yield tended to be higher in diet D2, followed by D3 and D1, respectively. Nevertheless, diet D3 tended to show higher dark meat and drumstick meat yield than D2 or D1. Dry meat yield was statistically similar among dietary groups. However, D1 tended to show the highest dry meat yield, followed by D2 and D3, respectively.
Table 3:Meat yield traits (%) of broiler fed diets with Bacillus subtilis and Spirulina platensis at 35 days of age. +D1=Control diet; D2=Control diet supplemented with Bacillus subtilis; D3=Control diet supplemented with Spirulina platensis; NS, p>0.05; *, P˂0.05; **, p˂0.01

Diet D1 was found to be better than D2 or D3 in terms of dressed meat and heart weight in which D2 and D3 were almost similar for these traits. The other meat yield traits and live weight did not differ among the dietary groups which contradicted Kaoud [24] and Molner et al. [31]. Kaoud [24] reported an improved carcass yield in the diet with (Spirulina Platensis 1kg/ton of feed). Molner et al. [31] showed improved carcass and thigh meat in the diet with Bacillus Subtilis over the control diet. However, in this study, the diet with Spirulina Platensis (D3) and the diet with Bacillus Subtilis both showed a lower carcass yield in comparison with the control diet which also contradicted Joya et al. [30]. They reported the improved carcass yield in the diet with Spirulina Platensis and Bacillus Subtilis at 42 days of age of the broiler chickens. It has also been found in this study that the control diet (D1) tended to be better than diet D2 or D3 in terms of the percentage of dry meat yield. Of the two diets, diet D2 tended to be better than D3 in terms of the dry matter percentage of meat yield. No previous literature was found on the percentage of the dry meat yield affected by Spirulina Platensis or Bacillus Subtilis.
Lipid profiles of broiler chickens fed diets with Bacillus Subtilis and Spirulina Platensis
Lipid profiles: Total Cholesterol (TC), Triglycerides (TG), High- Density Lipoprotein (HDL), and Low-Density Lipoprotein (LDL) were statistically similar among the diets (p˃0.05) (Table 4). However, diet D2 tended to show the lowest amount of TC and LDL and the highest amount of HDL, correspondingly in D1 and D3, respectively.
Table 4:Lipid profiles of broiler fed on diets with Bacillus subtilis and Spirulina platensis at 35 days of age. +sup>D1=Controldiet; D2=Control diet supplemented with Bacillus subtilis; D3=Control diet supplemented with Spirulina platensis; NS, p>0.05; TG=Triglycerides, HDL=High-density lipoprotein, LDL=Low-density lipoprotein.

Despite there being no significant difference among the diets for the blood lipid profiles of the birds, diet D2 was found to be the best among the diets because it contained the lowest amount of blood TC and LDL and the highest amount of HDL. Of the rest diets, diet D1 performed better than diet D3 in terms of the blood lipid profiles of the birds. Therefore, it is assumed that diet D2 may be the most suitable diet among the dietary groups in terms of the blood lipid profiles of the birds, corroborated by Aliakbarpour et al. [32]. They found a lower amount of TG, LDL, and TC and higher amount of HDL in the diet supplemented with Bacillus Subtilis (50g/kg of feed) over the control diet. The present study is also consistent with Santroso et al. [12]. They showed a lower amount of blood cholesterol and TG of the birds in the diet supplemented with Bacillus Subtilis (1%). Joya et al. [30] reported that Bacillus Subtilis (0.05%) affected to decrease TC and TG in the blood serum of broilers.
Conclusion
The present study reveals that diet D2 (Bacillus Subtilis @0.5g/ kg diet) may be the best-performer dietary group among the diets considering the growth and blood lipid profiles (total cholesterol, LDL, and HDL) of the bird. Meat yield traits except for dressing yield and heart weight were statistically similar among the dietary groups. Diet D1 (control) showed higher dressing yield and heart weight compared to diet D2 or D3. Hence, D2 was comparable to D3 for meat yield traits. Diet D2 had the lowest amount of blood cholesterol and low-density lipoprotein, and the highest amount of high-density lipoprotein among the dietary groups, followed by D1 and D3, respectively. Therefore, D2 (0.5g Bacillus Subtilis/ kg diet) may be considered a beneficial dietary group among the diets for the growth, meat yield traits, and blood lipid profiles of the broilers. However, more studies are needed using Bacillus Subtilis and Spirulina Platensis at different levels in the diet to produce safe and profitable broilers.
Acknowledgment
The authors are grateful to the Department of Dairy and Poultry Science, Faculty of Veterinary Medicine and Animal Science, BSMRAU, Gazipur-1706, Bangladesh, for allowing us to operate field and lab-based research works.
References
- Whitehead CC (2002) Nutrition and poultry welfare. World's Poultry Science Journal 58(3): 349-356.
- Sorum H, Sunde M (2001) Resistance to antibiotics in the normal flora of animals. Veterinary Research 32(3-4): 227-241.
- Burgat V (1999) Residues of drugs of veterinary use in food. Rev Prat 41(11): 985-990.
- Andremont A (2000) Consequences of antibiotic therapy to the intestinal ecosystem. Ann Fr Anesth Reanim 19(5): 395-402.
- Barton MD (1998) Does the use of antibiotics in animals affect human health? Aust Vet J 76(3): 177-180.
- Patterson JA, Burkholder KM (2003) Application of prebiotics and probiotics in poultry production. Poult Sci 82(4): 627-631.
- Haghighi HR, Gong J, Gyles CL, Hayes MA, Zhou H, et al. (2006) Probiotics stimulate production of natural antibodies in chickens. Clin Vaccine Immunol 13(9): 975-980.
- Park JH, Yun HM, Kim IH (2018) The effect of dietary Bacillus subtilis supplementation on the growth performance, blood profile, nutrient retention, and caecal microflora in broiler chickens. Journal of Applied Animal Research 46(1): 868-872.
- Zhang ZF, Kim IH (2014) Effects of multistrain probiotics on growth performance, apparent ileal nutrient digestibility, blood characteristics, cecal microbial shedding, and excreta odor contents in broilers. Poultry Science 93(2): 364-370.
- Cartman ST, Ragione RML, Woodward MJ (2008) Bacillus subtilis spores germinate in the chicken gastrointestinal tract. Appl and Environ Microbiol 74(16): 5254-5258.
- Sogaard H, Demark TS (1990) Microbials for feed: Beyond lactic acid bacteria. Feed International 11: 33-37.
- Santroso U, Ohtani S, Tannaka K, Sakaida M (1999) Dried Bacillus subtilis culture reduced ammonia gas release in poultry house. Journal of Animal Science 12(5): 806-809.
- Wang WC, Yan FF, Hu JY, Amen OA, Cheng HW (2018) Supplementation of Bacillus subtilis-based probiotic reduces heat stress-related behaviors and inflammatory response in broiler chickens. J Anim Sci 96(5): 1654-1666.
- Lei ZH (2011) Analysis of the major organic acid in Fenjiu and identification of acid-producing bacteria. Liquor Making Science Technology 38: 24-28.
- Latorre JD, Hernandez VX, Kallapura G, Menconi A, Pumford NR, et al. (2014) Evaluation of germination, distribution, and persistence of Bacillus subtilis spores through the gastrointestinal tract of chickens. Poultry Science 93(7): 1793-1800.
- EFSA FEEDAP Panel (EFSA Panel on Additives and Products or Substances used in Animal Feed) (2015) Scientific opinion on the safety and efficacy of Bacillus subtilis PB6 (Bacillus subtilis) as a feed additive for laying hens and minor poultry species for laying. European Food Safety Authority 13(1): 3970.
- Tactacan GB, Schmidt JK, Miille MJ, Jimenez DR (2013) A Bacillus subtilis spore-based probiotic for necrotic enteritis control in broiler chickens. Journal of Applied Poultry Research 22(4): 825-831.
- Vonshak A (1997) Spirulina platensis (Arthrospira) physiology cell-biology and biotechnology. Journal of Applied Phycology 9: 295-296.
- Parikh P, Mani U, Iyer U (2001) Role of spirulina in the control of glycemia and lipidemia in type 2 diabetes mellitus. Journal of Medicinal Food 4(4): 193-199.
- Khan M, Shobha JC, Mohan IK, Naidu MUR, Sundaram C, et al. (2005) Protective effect of Spirulina against doxorubicin‐induced cardiotoxicity. Phytotherapy Research 19(12): 1030-1037.
- Ross E, Dominy W (1990) The nutritional value of dehydrated, blue-green algae (Spirulina Plantensis) for poultry. Poultry Science 69(5): 794-800.
- Gruzauskas R, Lekavicius R, Raceviciute SA, Sasyte V, Tevelis V, et al. (2004) Optimizing the digestion process in broiler chickens with symbiotic preparations. Veterinary and Zootechnics 28(50): 51-56.
- Inborr J (1998) Haematococcus, the poultry pigmentor. Feed Mixing 6(2): 31-34.
- Kaoud HA (2015) Effect of Spirulina platensis as a dietary supplement on broiler performance in comparison with prebiotics. Specialty Journal of Biological Sciences 1(2): 1-6.
- Kharde SD, Shirbhate RN, Bahiram KB, Nipane SF (2012) Effect of spirulina supplementation on growth performance of broilers. Indian Journal of Veterinary Research 21(1): 66-69.
- Onunkwo DN, Okoro IC (2015) Egg production performance of three varieties of guinea fowls in humid tropics. International Journal of Current Research and Review 7(8): 1-6.
- Liu X, Yan H, Lv L, Xu Q, Yin C, et al. (2012) Growth performance and meat quality of broiler chickens supplemented with Bacillus licheniformis in drinking water. Asian-Australas J Anim Sci 25(5): 682-689.
- Vimon S, Angkanaporn K, Nuengjamnog C (2020) Evaluation of dietary probiotic (Bacillus subtilis KMP-BCP-1 and Bacillus licheniformis KMP-9) supplementation and their effects on broiler chickens in a tropical region. Journal of Applied Animal Research 48(1): 365-371.
- Rawshon ABM, Akanda RM, Rahman M, Hossain A, Islam S (2015) Prebiotic competence of spirulina on the production performance of broiler chickens. Journal of Advanced Veterinary and Animal Research 2(3): 304-309.
- Joya M, Ashayerizadeh O, Dastar B (2020) Effects of spirulina (Arthrospira) platensis and Bacillus subtilis PB6 on growth performance, intestinal microbiota and morphology, and serum parameters in broiler chickens. Animal Production Science 61(4): 390-398.
- Molnár AK, Podmaniczky B, Kürti P, Tenk I, Glávits R, et al. (2011) Effect of different concentrations of Bacillus subtilis on growth performance, carcase quality, gut microflora and immune response of broiler chickens. Br Poult Sci 52(6): 658-665.
- Aliakbarpour HR, Chamani M, Rahimi G, Sadeghi AA, Qujeq D (2012) The Bacillus Subtilis and lactic acid bacteria probiotics influences intestinal mucin gene expression, histomorphology and growth performance in broilers. Asian Aust J Anim Sci 25(9): 1285-1293.
© 2024 Mohammad Aminul Islam. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






