- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Assessment of Urethane as an Anesthetic in Pekin Ducks
Amanda K Darbyshire1,2*, Gregory S Fraley3, Aidan Horvath1 and Jeff C Ko4
1Laboratory Animal Program, Purdue University, USA
2Department of Comparative Pathobiology, Purdue University, USA
3Department of Animal Science, Purdue University, USA
4Department of Veterinary Clinical Sciences, USA
*Corresponding author: Amanda Darbyshire, Purdue University, West Lafayette, IN, USA
Submission: November 29, 2023;Published: December 19, 2023

ISSN: 2576-9162 Volume9 Issue4
Abstract
Urethane is a long-acting anesthetic used in laboratory animals to undertake procedures where the preservation of neural transmission and autonomic reflexes is essential. There is little information about the quality of anesthesia induced by urethane in ducks. This study aimed to investigate the quality of urethane anesthesia in Pekin ducks. Urethane was administered IV bolus (1.25g/kg) to 9 ducks followed by continuous rate infusion (CRI, 0.5-2g/kg/hr) to attempt to achieve anesthesia for up to two hours. Time and duration to a defined depth of anesthesia as quantified by measurements of muscle relaxation, antinociception (toe pinch), cardiorespiratory, and eye reflexes were assessed every 5 minutes during the urethane CRI maintenance. In addition, air sac cannulation was attempted to assess a possible surgical plane of anesthesia. The time to the defined depth of anesthesia was 7.67±4.74 minutes. Paradoxical muscle movements with or without rigidity were observed before complete muscle relaxation and periodically after muscle relaxation had been achieved. Inconsistent absence of palpebral reflex and antinociception occurred in most of the ducks during the anesthesia period. Only 2 ducks reached the depth of anesthesia for air sac cannulation with one duck moving during the procedure. Three of the 9 tested ducks died before the study ended. We concluded that urethane at the dosages tested induced a reasonable immobilization for non-invasive procedures in Pekin ducks but was inadequate in producing a surgical plane of anesthesia for air sac cannulation surgery. Furthermore, it had a narrow margin of safety for producing the defined depth of anesthesia in the current study.
Keywords:Anesthesia; Urethane; Duck; Analgesia
Abbreviations:CRI: Constant Rate Infusion; TAS: Total Anesthesia Score
Introduction
Urethane is a long-acting anesthetic used in laboratory animals for procedures where the preservation of neural transmission and autonomic reflexes are essential. It is typically used in research investigating neuronal function in visual, somatosensory, and hippocampal cortical regions in mammals and birds [1,2]. Schumacher discovered that in urethaneanesthetized songbirds, intrinsic neural excitability decreases, but spectral tuning and single neuron discrimination remain unchanged compared to the awake state [2].
Advantages of using Urethane include minimal impact on sensory evoked responses and neuronal discharge, contrasting with other anesthetics that depress and alter these functions [1]. However, urethane has notable disadvantages. It was initially considered to have anti-neoplastic properties and was used in chemotherapy for human leukemia and multiple myeloma, but it led to side effects like leukopenia, nausea, vomiting, and hepatic necrosis [3]. Studies in mice and Drosophila suggest potential carcinogenicity, especially in repeated survival procedures, where it’s employed to induce pulmonary adenomas [3]. For these reasons, urethane is not recommended for survival procedures. Additionally, its lack of pharmaceutical grade availability can introduce batch variability, making pharmaceuticalgrade anesthetics the preferred choice.
Currently, urethane is primarily utilized in research studies related to sleep waves and neurophysiology, enabling data collection without hindering sensory responses as traditional anesthetics might [1]. At our institution, an investigator aimed to explore vagal reflexes in Pekin ducks. Urethane was considered for anesthesia during vagal nerve access, emphasizing minimal interference with vagal responses. Previous research, like Chen et al. [4] study on Beijing ducks, used urethane at 1g/kg IV for anesthesia before exsanguination, although its quality wasn’t assessed [4]. Urethane, despite its slow onset, offers prolonged anesthesia (8-24 hours) in mammals, but it can be toxic to rats, impacting vasculature, renal function, and body fluids when administered intraperitoneally [5].
There is little knowledge about the quality of anesthesia and antinociception that urethane produces in ducks. In this study, we investigated the ability of urethane to produce deep, reliable anesthesia and its antinociceptive property for non-survival surgical procedures in Pekin ducks.
Materials and Methods
Animals
Nine juvenile Pekin ducks (mean weight 4.40 ± 0.67 kg) of mixed sex were obtained from a local commercial producer (Maple Leaf Farm, Inc; Leesburg, IN USA). The ducks were healthy and housed in a poultry barn under production standards [6]. They were housed indoors on pine shaving bedding and fed standard adult, breeder duck diet [6]. This study was approved by the Purdue Institutional Animal Care and Use Committee, protocol 2109002191 (09-24- 2021).
Urethane Preparation
Urethane (Sigma-Aldrich; St. Louis, MO) was dissolved in sterile reverse osmosis water to a 0.5g/ml solution and filtered through a 20μm filter for sterilization within a chemical fume hood. It was prepared fresh for each procedure (Figure 1).
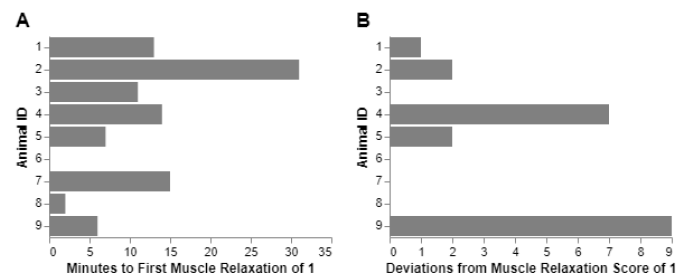
Figure 1:Latency to muscle relaxation (A) and muscle movement events (B) in 9 ducks anesthetized with urethane.
Study Design
Ducks were briefly hand-restrained for catheter placement into the medial metatarsal vein. A pilot study showed that a 1.25g/kg dose was needed to induce unconsciousness in Pekin ducks. The ducks were injected with urethane preparation starting with a 1.25g/kg bolus followed by constant rate infusion (CRI, 0.5-2g/kg/ hr) to attempt to achieve anesthesia. Time to general anesthesia was recorded with a scale of total anesthesia score, which was defined as a lack of righting reflex, a muscle relaxation score (MRS) of 1, a palpebral reflex score of 2, and a lack of response to nociception as elicited by a moderate toe pinch (Table 1). Muscle relaxation was assessed by jaw tone. Palpebral reflex was assessed by applying a stream of saline pressurized through a catheter to the eye. This was done to prevent potential trauma to the eye from repeated stimulation, which could occur by touching with a swab. Toe pinch was performed using plastic sponge forceps at the mid-metatarsus to the third rachet. Anesthesia was monitored including heart rate, respiratory rate, rectal body temperature, ECG, toe pinch, palpebral reflex, and muscle relaxation. The scores for respiration pattern (spontaneous, periodic, or apneic), muscle relaxation, palpebral reflex, and antinociception scores from Table 1 were added together to determine a total anesthesia score, where a score of 3 was considered ideal (Table 2). The parameters were obtained every 5 minutes throughout the procedure. Urethane CRI was continued for up to 2 hours, then an IV injection of Euthasol (1ml/10lb) (Virbac; Fort Worth, TX) was given to euthanize the duck at the end of the study.
Table 1:The anesthetic scoring system used in this study based on various degrees of respiration, muscle relaxation, and palpebral reflex. Each column was summed to create a combined total anesthesia score.
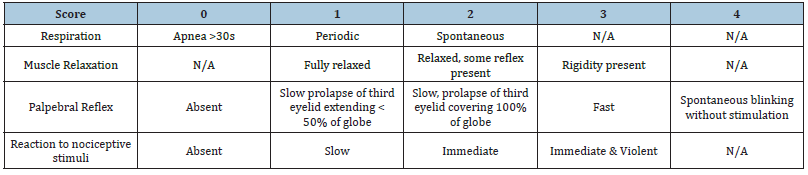
Table 2:Total Anesthesia Score (TAS), which is a combined score from the anesthetic score system defined in Table 1.

Additional antinociception assessment: If an appropriate anesthetic plane (TAS 3-4) was achieved and sustained for at least 15 minutes during the urethane CRI maintenance an air sac cannulation procedure was attempted and the nociceptive reaction was assessed. For air sac cannulation, an 11-blade was used to create a small skin incision over the abdominal air sac, located between the last rib and pelvis, and a 3-5mm diameter endotracheal tube was pushed into the body wall to cannulate the abdominal air sac with an aid of a hemostat.
Data analyses
This study represents an attempt to characterize the expected responses of ducks to urethane anesthesia over the time course of a typical procedure. Because we did not examine any discrete treatment groups, group differences were not analyzed, but rather the physiological response for each animal is reported to provide an overview of anesthetic effects. For time course analyses (Figures 2 & 3), all datapoints from each animal were combined to construct a polynomial regression line to visualize overall trends. Regression summaries are reported, but due to low r^2 values caused by small samples, these regressions are primarily intended as visual aids, and not for prediction.
Result and Discussion
Time to general anesthesia
The average time to general anesthesia was 7.67 ± 4.74 minutes. The total anesthesia time and dosing regimen for the maintenance of anesthesia is shown in Table 3.
Table 3:Dosing regimen, amount of urethane used for maintaining general anesthesia, and total anesthesia time in 9 ducks.
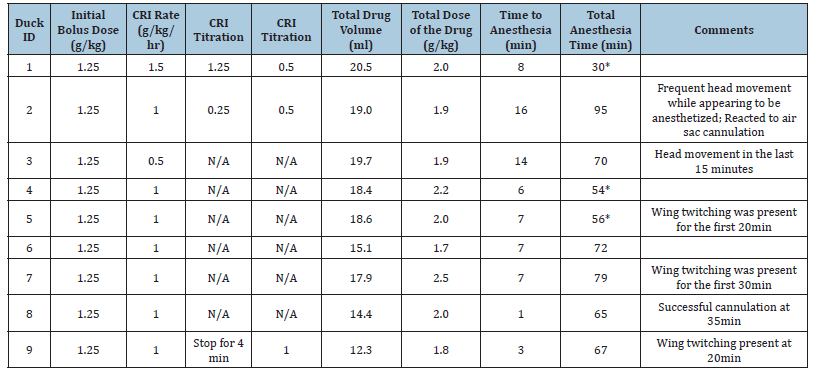
Table Abbreviations: CRI, continuous rate infusion; TAS, total anesthesia score.
Heart rate and body temperature
The average heart rate ranged from 135 to 247 (mean=204.09, std = 41.11)bpm. Electrocardiogram results were normal throughout the anesthetic period except for those animals which died. Just prior to death, ECG abnormalities were present. Body temperature was maintained at an average of 103 to 106 (mean=14.47, std = 1.48) degrees Fahrenheit.
Respiration
All the ducks maintained spontaneous respiration. The average respiration rate for individuals was between 10 to 24 (overall mean=17.47, std=9.48) breaths per minute. For the 3 ducks that died during anesthesia, apnea occurred 3 minutes before their deaths, despite endotracheal intubation with assist ventilation after apnea.
Muscle relaxation
An average of 11 minutes (std=9.17) minutes after urethane CRI was required to reach full muscle relaxation. However, most ducks experienced spontaneous muscle movement events before full muscle relaxation with an average of 2 events per animal that included muscle movements or rigidity after initially reaching a muscle relaxation of score 1 (Figure 1).
Palpebral reflex
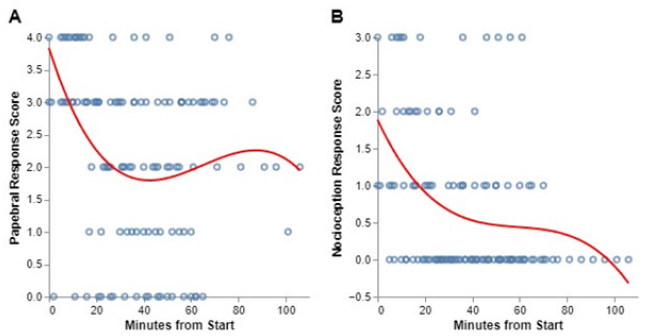
The presence of a palpebral reflex was highly variable but was usually maintained throughout anesthesia (Figure 2A).
Nociception
The nociceptive response to toe pinch was highly variable in both intensity and presence throughout anesthesia (Figure 2B). At times there was no response, while at the next reading it occasionally would return.
Figure 2:Timelines of palpebral (A) and nociceptive (B) responses in 9 ducks anesthetized with urethane.
Air sac cannulation
Only 2 ducks reached a stable anesthetic plane of 3-4 suitable for air sac cannulation tests. One of these ducks had an immediate reaction to the skin incision and further attempt at the procedure was terminated and the animal was humanely euthanized. The other duck had no response to the cannulation and the procedure was successfully completed.
Total anesthesia score
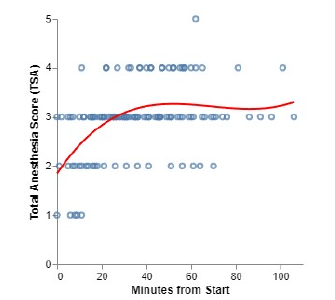
Total anesthesia scores averaged 2.97 (std=0.77) indicating a moderate plane of anesthesia. Only one duck reached a score of 5, which was profound anesthesia (Figure 3). A 3rd degree polynomial regression was applied to data from all ducks over approximately 100 minutes, resulting in adjusted R-squared values of 0.115 for the palpebral reflex (y=3.8-0.1+0.002x2-0.00001x3), 0.148 for nociception (y=1.9-0.07+0.001x2-0.000006x3), and 0.215 for the total anesthesia score (y=1.8+0.07-0.001x2-0.000005x3).
Figure 3:Timeline to achieve different anesthetic planes of urethane anesthesia in 9 ducks. Time started after urethane intravenous bolus injection, followed by the CRI regimen shown in Table 3.
Discussion
The purpose of our study was to evaluate the efficacy of urethane anesthesia for non-survival procedures on Pekin ducks for research since urethane is known to induce a surgical plane of anesthesia with minimal effects on both central and peripheral nervous neurotransmission in laboratory animals [7]. We found that urethane produced immobilization and variable planes of anesthesia in ducks but did not provide sufficient anesthesia to prevent response to surgical stimulation.
To prevent the risk of fatality associated with a fast urethane bolus injection [8], We investigated an approach involving an initial bolus followed by continuous infusion to maintain anesthesia in ducks. Our findings revealed a slow onset of anesthesia, taking an average of 7.67 ± 4.74 minutes, considerably longer than typical fast-acting intravenous injectable anesthetics. Moreover, the ducks needed physical restraint before full immobilization, causing potential stress to the birds and additional labor for personnel. On the other hand, the heart and respiratory rates and body temperature largely remained within normal ranges during the anesthesia. Even when the heart rate decreased, it was still maintained above the defined bradycardia criteria (<50% of the awake baseline). Spontaneous respirations were relatively well maintained throughout anesthesia until a deep plane of anesthesia was reached. Before this deep plane of anesthesia, there was no need to provide oxygen supplementation or ventilation assistance in these ducks.
In the current study, maintaining the desired anesthetic depth with urethane proved challenging. If it reached an excessively deep level, rapid cardiorespiratory depression occurred, which seemed difficult to reverse. In one duck in our study, urethaneinduced profound depression led to apnea. Even with attempts to halt urethane infusion, start cardiopulmonary resuscitation, and provide ventilation support, the duck succumbed within minutes despite these efforts.
Urethane induced variable degrees of muscle relaxation in the duck. It took an extended period (~12 minutes) for these ducks to achieve full muscle relaxation and once achieved, it was not sustained. These ducks would frequently exhibit paradoxical muscle movements consisting of wing tremors, and head and neck movements with or without muscle rigidity during the anesthetic period, even though the duck appeared to be well anesthetized. Another interesting note was that the palpebral reflex was mostly maintained and was not a useful indicator of anesthesia for this study.
To assess urethane induced antinociceptive property, we first used plastic sponge forceps to inflict a nociceptive response with a toe pinch. We found the nociceptive response was highly variable and without obvious patterns during the anesthetic period. The nociceptive response was difficult to interpret with the duck’s behavioral response during the anesthesia. Often, when the reflex was absent the animal would still display muscle movements, such as moving the entire head and neck. The question was raised whether the plastic sponge forceps used were too flexible for the consistent force applied to the toe. A pair of metal hemostats were also used for extra tests and found to elicit the same inconsistent result as observed with the plastic sponge forceps. Urethane’s property of inconsistent antinociception was further confirmed through our air sac cannulation test. Only two ducks met the predefined anesthetic depth criteria, including full muscle relaxation and lack of palpebral reflex, for air sac cannulation attempts. One duck showed no reflex or other movements for a successful cannulation and the other duck had an immediate reaction upon skin incision. Although urethane has been used for anesthesia and surgery in many other laboratory animals [8-10], we found that the surgical plane of anesthesia was difficult to achieve in Pekin ducks with urethane in this study.
We found several challenges in using urethane as an anesthetic in ducks. We administered urethane with an initial bolus followed by CRI and allowed most of the ducks to achieve an anesthetic depth score of 2 to 4, which indicated a plane of anesthetic depth ranging from light to deep. However, one of the 9 ducks reached an anesthetic depth score of 5 which was classified as a very deep plane, and 3 of the 9 ducks died before the end of the study. These results indicate high intra-breed variations in response to urethane in these Pekin ducks. The nonpharmaceutical grade use of urethane could also have resulted in variability between batches, which were mixed fresh for each procedure, and may have been a limitation in this study.
Another challenge was that the time took to achieve the moderate plane of anesthesia was relatively long and the CRI urethane dose was difficult to adjust to maintain a consistent depth of anesthesia once these ducks were immobilized. A similar difficulty in maintaining a stable plane of anesthesia was observed in our pilot study, where several bolus doses of urethane had to be given and repeat dosing was needed approximately every 15-25 minutes. A similar challenge was reported in urethane-anesthetized pigeons [11]. In contrast to these avian species, it has been reported that a single 1.2g/kg dose in rats achieved over 24hr of anesthesia without the need of repeated dosing [8].
Conclusion
In summary, our study demonstrated that administering a 1.25g/kg intravenous bolus followed by a continuous rate infusion of 0.5-1g/kg/hr offers the most effective approach for immobilizing juvenile Pekin ducks. However, it’s important to note that even under this anesthesia regimen, occasional movements were observed. These movements have the potential to impact noninvasive monitoring procedures such as electroretinograms, electroencephalograms, and electromyograms.
Moreover, our findings revealed several disadvantages associated with urethane anesthesia, including challenges in maintaining a stable plane of general anesthesia, poor antinociceptive properties, and a narrow margin of safety. These factors collectively contribute to the conclusion that urethane may not be the ideal choice for surgical procedures in Pekin ducks, despite its advantages.
Acknowledgements
We would like to acknowledge Dr. Lee Matthews, Carol Dowell, Julie Brown, and Katy Harringer for their technical skills and intense involvement with this project.
References
- Sorrenti V, Cecchetto C, Maschietto M, Fortinguerra S, Buriani A, et al. (2021) Understanding the effects of anesthesia on cortical electrophysiological recordings: A scoping review. International Journal of Molecular Sciences 22(3): 1286.
- Schumacher JW, Schneider DM, Woolley SMN (2011) Anesthetic state modulates excitability but not spectral tuning or neural discrimination in single auditory midbrain neurons. Journal of Neurophysiology 106(2): 500-514.
- Field KJ, Lang CM (1988) Hazards of urethane (ethyl carbamate): A review of the literature. Laboratory Animals 22(3): 255-262.
- Chen Y, Lin D, Ohmori Y, Naito J (1997) Distributions of the Cardiac Plexuses and Ganglia in the Beijing Duck. Journal of Veterinary Medical Science 59(5): 409-411.
- Severs WB, Keil LC, Klase PA, Deen KC (1981) Urethane Anesthesia in Rats. Altered ability to regulate hydration. Pharmacology 22(4): 209-226.
- Chen X, Shafer D, Sifri M, Lilburn M, Karcher D, et al. (2021) Centennial Review: History and husbandry recommendations for raising Pekin ducks in research or commercial production. Poultry Science 100(8): 101241.
- Maggi CA, Meli A (1986) Suitability of urethane anesthesia for physiopharmacological investigations in various systems Part 1: General considerations. Experientia 42(2): 109-114.
- Field Karl, White WJ, Lang CM (1993) Anaesthetic effects of chloral hydrate, pentobarbitone and urethane in adult male rats. Lab Anim 27(3): 258-269.
- Fish RE (1997) Pharmacology of Injectable Anesthetics. In: Kohn DF, Wixson SK, White WJ, Benson GJ (Eds.), Anesthesia and Analgesia in Laboratory Animals. Academic Press, San Diego, USA, pp. 1-28.
- Lipman NS, Marini RP, Flecknell PA (1997) Anesthesia and Analgesia in Rabbits. In: Kohn DF, Wixson SK, White WJ, Benson GJ (Eds.), Anesthesia and Analgesia in Laboratory Animals. Academic Press, San Diego, USA, pp. 205-232.
- Tisdale RK, Tieri L, Rattenborg NC, Beckers GJL, Lesku JA (2018) Spectral properties of brain activity under two anesthetics and their potential for inducing natural sleep-in birds. Front Neurosci 12: 881.
© 2023 Amanda K Darbyshire. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






