- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Investigation of Drug Resistance of Common Drugs to Eimeria Species Isolated in Field Conditions from Broiler Flocks in Mazandaran Province in Iran
Arash Ramezanpour1* Parvaneh Nafis Fard1 Golamreza Rami2 Golam Ali Kalidari1
1Faculty of Veterinary Medicine, Department of Clinical Sciences, Ferdowsi University of Mashhad, Iran
2Faculty of Veterinary Medicine, Department of Pathobiology, Ferdowsi University of Mashhad, Iran
*Corresponding author: Arash Ramezanpour, Faculty of Veterinary Medicine, Department of Clinical Sciences, Ferdowsi University of Mashhad, Iran
Submission: August 01, 2023;Published: October 02, 2023

ISSN: 2576-9162 Volume9 Issue4
Abstract
Introduction: Coccidiosis is one of the most common and important diseases of the poultry industry,
which is caused by Eimeria protozoa and mostly occurs in the form of enteritis. Today, due to the excessive
use of coccidiostat drugs, Eimeria species have developed drug resistance to these drugs, and this has
economic consequences.
Methods: In the present study, litter samples were collected from broiler farms with a history of coccidiosis
in Mazandaran province. Eimeria oocysts from infected samples were cultured and propagated in a 2.5%
potassium dichromate solution in a humid incubator at 27 °C, and the sporulated oocysts were counted
and washed for propagation in broiler chickens. Then, 60 broilers of Ross breed were divided into 3
groups. (Maduramycin group, positive control group, negative control group) From the beginning of the
study, maduramycin drug was added to the diet of the maduramycin group for 30 days. On the fourteenth
day, 200,000 mixed oocysts of Eimeria species were given orally to each bird. Daily litter samples were
collected from the 5th day to the 14th day after infection, and the OPG of the litter of each group was
determined using the McMaster method. In addition, chickens of each group were weighed daily until the
end of the study.
Discussion and conclusion: Based on the results of this study, the presence of drug resistance of Eimeria
species to maduramycin in the broiler farms of Mazandaran province is confirmed and reported (because
there was a significant difference between the OPG of the maduramycin group and the positive control
group (P<0.05).) It is necessary for the health authorities of the country’s poultry industry to look for a
solution to solve this health problem so that more economic damage is not caused to the industry and the
country’s economy.
Keywords:Coccidiosis; OPG; Drug resistance; Maduramycin; Mazandaran; Salinomycin
Introduction
Coccidiosis is one of the most common and important diseases of the poultry industry, which is caused by the protozoan of the Eimeria genus and occurs mostly in the form of enteritis [1-3]. Coccidia are a broad group of single-celled parasitic organisms in the Protozoa suborder and the Epicomplexa order. As a group, the Eimeria genus mainly has a specific host, which means that each Eimeria species involves a specific host or a group of hosts that are closely related to each other [1]). A severe infection caused by coccidia that causes clinical manifestations of the disease is called coccidiosis, and a brief infection that does not lead to inconsiderable clinical effects is called coccidiosis [4]. So far, out of 9 known Eimeria species in chickens, only 7 species have been diagnosed as pathogenic [5]. Among the reported species, Eimeria bronti, Eimeria nectrix, Eimeria tenella have high pathogenicity and contagiousness along with blood lesions, and Eimeria maxima and Eimeria acerulina species have moderate pathogenicity and usually cause subclinical coccidiosis in meat herds [6,7]. The identification and confirmation of two other species that have been repeatedly mentioned in the literature (Imria Hagani and Miwati) are under investigation [8]. Most of the age of fighting with Imeria with casualties is from 3 to 18 weeks old [9].
Inside the body of each host, there may be several species of Eimeria with different pathogenic powers, while they are completely specific to that host. Infection with Eimeria species is everywhere and it is said that the only limitation in their distribution is the limitation in the distribution of their host [10-20]. Of course, the disease occurs mostly in conditions of high density of birds in the litter system, which is suitable for the growth of pathogenic parasites. Therefore, coccidiosis is important in dense broiler breeding centers [6]. In addition to causing the disease, subclinical contamination has weakened the food conversion ratio, and since 70% of the cost of broiler breeding is related to providing feed, the economic effects of coccidiosis will be very significant. For example, the total economic loss caused by poultry coccidiosis in England in 1995 was estimated at 38.5 million pounds [21]. For this reason, a lot of research has been done to prevent and treat this form of the disease, and the most important success in this field is the discovery of coccidiostat drugs, especially ionophore compounds [10]. The continuous administration of coccidiostats in the diet has reduced or stopped the clinical form of the disease. In the long term, due to the non-specific effect of these drugs on Eimeria acerulina and Maxima species, the ground has been prepared to accelerate the development of drug resistance in these species compared to other species. This led to the emergence of subclinical coccidiosis at the level of broiler chicken farms [11].
Today, due to the economic losses caused by the spread of subclinical coccidiosis in broiler flocks, detailed studies have been done on the pathogenicity of different species. [12], these studies include rapid diagnosis methods [11,12], disease epidemiology [13-15], the possibility of drug resistance in these species [13,16], treatment and prevention of subclinical coccidiosis. In this regard, the aim of this study is to investigate the drug resistance of common drugs to Eimeria species isolated in farm conditions from broiler chicken flocks in Mazandaran province, so as to be an alarm and a warning for the consequences and damages of coccidiosis drug resistance.
Discuss
Drug resistance
Today, coccidiosis is known as an important and common disease that can cause a lot of economic losses in the poultry breeding system. Clinical coccidiosis increases the feed conversion ratio from 4 to 10%, and since 70% of the cost of broiler breeding is related to feed supply, the economic effects of coccidiosis will be very significant. For example, the total economic loss caused by poultry coccidiosis in England in 1995 was estimated at 38.5 million pounds [1]. Economically, this disease involves huge losses, which is unique in its kind, because in the world, this disease causes about 60 to 120 million dollars in financial damage, which is a good indication of the depth of the disaster. In addition to these expenses, which are spent annually in the world to purchase medicine, treatment or prevention of coccidiosis, it is an amazing figure of 150 million dollars [20]. In this sense, disease control and prevention are one of the necessary and key measures to increase productivity in poultry farming [4,17]. Until now, many efforts have been made by researchers in the direction of knowledge and epidemiology of the disease, improvement of management methods, discovery and production of useful coccidiostats and research to prepare a suitable vaccine [12,18].
Administration of coccidiostat in the diet of broilers during the growth period is still used as the most practical way to control and prevent coccidiosis. Although this form of prevention has caused a significant reduction of clinical coccidiosis in the herd compared to a few decades ago, the continuous use of drugs has accelerated the development of drug resistance, which has many economic consequences.) [18]. Regarding the resistance of coccidiostat drugs in the world, I would like to draw your attention to some of these reports:
There are reports of resistance to amperoleum and amperoleum etopabate. Amprolium with the brand names of Amperol and Amprolium etopabat with the brand names of Amprolium Plus and Amprole-i are available in the market. The use of amprolium in low doses inhibits the absorption of thiamine in coccidia [21]. Amprolium is primarily effective in the stage of asexual reproduction of the parasite and also on the primary and secondary stages of schizonts [22].
The general and relative resistance against Amprolium has been reported in Imeria isolates collected from poultry farms [19,23,24]. In different studies conducted on field isolates of Eimeria acerulina, they had low sensitivity to Amprolium. The percentage of sporulation of oocysts obtained from treated birds (birds that took medicine) was comparable to the percentage of oocysts obtained from untreated birds (birds that did not receive medicine) [25]. The studies conducted in the laboratory showed that the development of drug resistance against Amprolium even during the continuous use of its low doses in broiler chickens takes place very slowly and relatively [26,27].
In 1975, McLaughlin et al. [28], using 4 anti-coccidiosis drugs consecutively in broilers, succeeded in producing a strain of Eimeria tenella resistant to a dose of 125ppm of amprolium, and this state was created after 10 times of alternating use of the drug. Also, similar results were reported by Tamas et al. [29]. In a study conducted by Jeffers [30] and other researchers in 1974, it was found that isolates of Eimeria acerulina and Tenella that were isolated from the field showed resistance to the combination of 125ppm amprolium and 4ppm etopabat [30].
In 1973, Jeffers & Chali [31] showed that resistance to clopidol in two laboratory strains of Eimeria acerulina led to increased sensitivity to 4-hydroxyquinoline drugs. In an epidemiology study conducted by Jeffers, the occurrence of resistance to decoquinates in Eimeria acervolina in a herd that was using clopidol was reported as 53% [32].
In a study in North India by Anish Yadav and Gupta about the drug resistance of Eimeria tenella species isolated from the field with two of the common anticoccidiosis drugs Maduramycin and Salinomycin. In this study, 140 Ross breed chickens were examined in 10 groups, and for each bird, 105 oocysts isolated from the field were used to cause experimental infection, and finally resistance to the desired drugs was measured using the Global Index. They did not observe any drug resistance [33].
Now it can be concluded that the increase of information in the field of coccidiosis, risk factors, common treatments and effectiveness of drugs can play an important role in preventing drug resistance and subsequently reducing macroeconomic losses. For the first step, one should obtain detailed information about common treatments and their effectiveness in order to recognize inappropriate cases of using these drugs; Because as it was said, improper use of coccidiostats is the most important cause of resistance to these drugs [34].
To control and prevent this disease, the best thing to do is to disinfect the poultry house using methods such as flame throwing and using ammonia and lime releasing substances, and to clean the place from contamination, the second thing is to control the humidity of the bed between 20 and 30%. Adjusting the food ration and adding preventive drugs with appropriate doses in food mixers and using drugs with a known expiration date and without pharmaceutical adulteration are also taking into account that in the fourth and fifth weeks of breeding, the contamination of oocysts in the bed is high. At this time, adding coccidiocid drugs with a therapeutic dose is very appropriate [4,17]. In the case of coccidiostatic drugs that we use throughout the breeding period, prevention and control strategies should be used.
Materials and Work Methods
By referring to 10 different pharmacies in Mazandaran province from March 25, 2020, to April 20, 2021, and by completing the questionnaire and obtaining information from them, we identified the common drugs in this province and used the same drugs to measure resistance. According to the field research of poultry farms and also the experts of Mazandaran Province Veterinary Department, common coccidiostat drugs in the north of the country are Salinomycin, Maduramycin and Diclazuril respectively.
By referring to the broiler flocks and obtaining information about the history of the disease and the use of anti-coccidiosis drugs, he collected some of the substrate and after mixing, poured 9grams of it into the sample container and added 126ml of water to it. and remained in the laboratory for one night. The next day, we shake the glass containing the substrate and pass it through a strainer and centrifuge 15ml of the strained solution at a speed of 1500rpm for 5 minutes.
Then empty the supernatant and mix the resulting sediment in 15ml of saturated salt and centrifuge again, then remove some of the supernatant with the help of a Pasteur pipette and fill the chambers under the McMaster slide. Then the floating oocysts of each house are counted, and the average obtained is multiplied by 100, and thus the number of oocysts per gram of substrate is determined [1,3].
Oocysts were isolated from infected chicken farms and cultured in 2% potassium dichromate solution, and after washing and purification, they were fed to two-week-old chickens, and stool samples were taken daily for two weeks after that. This allows us to have young oocysts and increase the number of oocysts for drug evaluation. Then the stool samples of these chickens were taken, washed and cultured with a centrifuge, and the oocysts after sporulation were kept in the refrigerator and in potassium dichromate solution until the time of drug evaluation [35]. According to Table 1, five groups of chickens were selected.
Table 1:Study grouping.
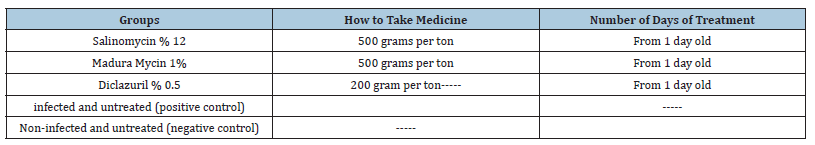
Then, the oocysts that were sampled from breeding farms and the processes of separation, purification and cultivation in 2% potassium dichromate solution were performed on them, were examined for sporulation. When the sporulation was confirmed by observing the sporocysts inside the oocyst under the microscope, it was again prepared to be fed to chickens by centrifugation twice and through washing and purification steps. Then, 200,000 sporulated Eimeria (equivalent to half a milliliter of the purified solution) of the propagated species were fed to the groups from the second week of breeding on June 27, 2016, by dropper [36,37].
In the treatment groups, despite the prescription of drugs, if Eimeria is observed, resistant species will be identified based on morphological characteristics (measurement of the length and width of the oocysts under the microscope). Then, using Spss software version 24, each drug group with positive and negative control group was statistically analyzed with independent t-test (in OPG of groups) and analysis of variance (in weighting).
Local stability of non-existence dog rabies disease equilibrium point E0
Result
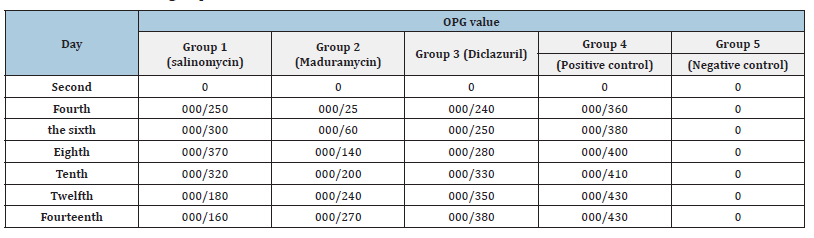
OPG results of the groups of all 5 groups during sampling days are shown in Table 2. There was no significant difference in the average number of oocysts counted in the different treated groups compared to the positive and negative control groups during the different days of the t-test (P<0.05).
Table 2:OPG results of groups.
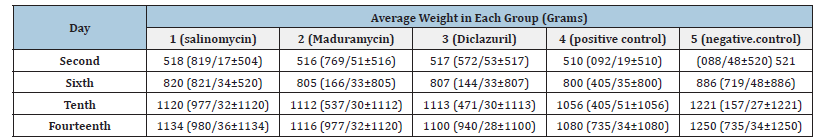
After creating experimental pollution and simultaneously with sampling, weighing was done, the results of which are shown in Table 3. Changes in weight loss in treatment groups and positive and negative control groups were compared. In this study, statistical analysis with one-way analysis of variance method did not show any significant difference in the weight of the treatment groups and the positive control group (P>0.05). While the weight loss changes in the treatment groups with the negative control group were highly significant (P<0.001), this amount of weight loss can cause high economic damage in a broiler poultry farm.
Table 3:Average weight in each group.
Conclusion
Administering coccidiostat in the diet of broilers during the growth period is still used as the most practical way to control and prevent this disease. Although this form of prevention has caused a significant reduction of coccidiosis in the herd compared to a few decades ago, the continuous use of the drug has accelerated the phenomenon of drug resistance and drug residue in chicken meat, both of which cause concerns.
In this study, the presence of drug resistance in Eimeria species against some common coccidiostat drugs used in the diet of broiler chickens in Mazandaran province was studied. For this purpose, sampling and field research were carried out from broiler farms in Mazandaran province, and OPG measurements of litter, carcass waste and weighing were used to determine resistance, and finally, resistant species were also identified.
The first drug group studied was salinomycin. This drug is available in the market under the brand names of Biocox, Coxystat, Sacox, Salgin and Salusin. For the first time, salinomycin was isolated from Streptomyces albus in soil samples collected in Japan [38,39]. This compound is a polyether carboxylic acid antibiotic that selectively plays a role in the transfer of monovalent alkaline cations such as Cs+, K+, Na+ and Rb+ [40,41]. The studies conducted on Eimeria acerulina, Maxima and Tenella indicate that salinomycin has a lethal effect against sporozoites, schizonts and the final stages of their schizogony [42,43].
Comparison and statistical analysis of OPG counting results in the salinomycin treatment group with the positive control group indicated the occurrence of resistance phenomenon in common Eimeria against this drug [44].
In 1998, in a comparative study between different coccidiostat drugs, Daugshis et al. [43] in Hanover, Germany showed the efficacy of salinomycin to control coccidiosis. In this study, the drug salinomycin had the best effect among other drugs, and all Eimeria species were sensitive to it, and no resistance was developed, which was suggested to be the reason for the use of coccidiostat drugs in a schedule and change in each period [45].
In another study, Chapman [44] in 1986 in America showed that Eimeria tenlai species isolated from meat farms have absolute resistance to the drug salinomycin and other drugs he studied, and the reason for this was the lack of use of anti-coccidiosis vaccine and new coccidiostat drugs [46,47]. By comparing these studies with the present study, it can be seen that the indiscriminate and unplanned use of salinomycin due to its lower price than other drugs are one of the important factors in the development of drug resistance to salinomycin in this province.
The second studied group was Maduramycin. This drug is available in the market under the brand name Seagro. Maduramycin is a monoglycoside polyether ionophore drug. Maduramycin with doses of 5 to 7ppm was more effective than narasin and monensin in reducing injuries and casualties and maintaining performance in birds infected by Eimeria strains that were resistant to ionophores and had almost the same effect as salinomycin. In the studies conducted on the effect of the drug, it was determined that in order to achieve the best results against Eimeria tenella, this drug must be present in the diet at the beginning of contamination or conflict [48].
In the study of Anish Yadav in 2001 in India, the drug resistance of Eimeria species against the drug maduramycin was also conducted and no drug resistance was observed, and the reason for this was the appropriate and planned use of this drug in the Indian poultry industry [49].
Peek & Landman [46] also conducted studies on the resistance of salinomycin, maduramycin, diclazuril, narasin, lazalucid, nicarbazine, monensin and clopidol drugs in Germany in 1996, 1999 and 2001 and discussed the different pattern of resistance during 1996, 1999 and 2001. So that in 1996, sensitivity was seen only in the drugs Narasin, Nicarbazine and Maduramycin, and the rest of the drugs showed relative to absolute resistance. In 1999, all drugs showed resistance, and in 2001, only clopidol was sensitive and the others were resistant. These researchers attributed the reason for the difference in the resistance of these drugs during these years to the use or non-use of these drugs in certain periods when the price of these drugs fluctuated in the market [49]. Again, by comparing these studies with the present study, the first thing that came to mind is the excessive consumption of maduramycin in Mazandaran province, followed by the development of resistance, which can be a warning for the poultry industry of the province and the country. This drug is also used together with salinomycin due to its more reasonable price and availability in different periods of broiler breeding.
The third group studied was diclazuril, which is available in the market under the brand name Clinacox. The studies conducted by Moss et al. (1988, 1989) and Verhein et al. showed that diclazuril has a lethal effect on both sexual and asexual stages of Eimeria tenella, large schizonts of Eimeria nectrix and acerulina, gametocytes of Eimeria bronti and zygotes of Eimeria maxima [22]. Diclazuril’s effects against Eimeria acerulina, Maxima, Nekatrix, Mitis, Bronti and Tenella remain for several days after stopping the drug, and this effect is unique among all anticoccidiosis drugs and polyether ionophores [50]. In the present study, the drug resistance of Eimeria species against diclazuril has been established in absolute form.
In a 1994 study in Brazil, Cavazo and Di Fabio showed that fields that had previously used diclazuril as a coccidiostat were now resistant to this drug and that fields that had not used this drug until now were sensitive to this drug [51]. The research method of these researchers is completely similar to our study, and they reached this conclusion from OPG, necropsy waste and weighing.
In the study of Arab Khazaeli et al. in 2013, the presence of resistance of three Eimeria species isolated from the field against the drugs diclazuril, salinomycin and amprolium etopabat, representing the most common anti-coccidiosis drugs in Iran’s poultry industry, was investigated. In this study, sampling was done from the farms of Mazandaran and Hamedan, and after purifying and obtaining the oocysts, the resistance to these drugs was investigated experimentally by placing 480 chickens in 13 groups. Then, about 250,000 to 300,000 oocysts per bird were used to cause experimental infection, and then the resistance of the desired drugs was measured using Global Index, Anticoccidial Sesivity Test and Optimum Anticoccidial Activity. In this study, none of the species was completely sensitive to the selected anti-coccidiosis drug and all species showed a decrease in sensitivity or relative resistance to salinomycin. Also, there was obvious resistance to amprolium etopabat and relative to absolute resistance to diclazuril [40].
This study and the present study were both conducted in Iran and in different farms and with different methods and reached similar results that can confirm the face of drug resistance in Iran. The results of both studies as well as other studies stated about diclazuril drug indicate excessive and unplanned use of these drugs [52].
Regarding the weighting results of the groups, it can be seen that at the end of the period, the positive control group (infectious and untreated) weighs 170grams less than the negative control group (non-infectious). Also, salinomycin, maduramycin and diclazuril groups weighed 116, 134 and 150grams less than the negative control group, respectively. The results indicate that the presence of drug resistance, like the treatment group, can be effective in weight loss. In this study, no significant difference was observed in the weight of treatment groups and positive control groups (P>0.05). While the weight loss changes in the treatment groups with the negative control group were highly significant (P<0.001), this amount of weight loss can cause high economic damage in a largescale broiler chicken farm. For example, let’s assume what kind of economic damage it can cause in a 50,000-broiler chicken farm in a full breeding period.
The results obtained in this study may not fully show the drug resistance of Eimeria species against coccidiostat drugs used in broiler farms in the province. But it seems that the occurrence of drug resistance of Eimeria species in the absolute level compared to commonly used drugs is a serious warning that it is necessary for the health authorities of the country’s poultry industry to look for a solution to solve this health problem so as not to cause more economic damage to the industry and the country’s economy. Among the ways that can be considered by veterinarians in the poultry sector, we can mention the discussion of replacing new drugs and also the possibility of coccidiosis vaccination in broilers. Also, the veterinary organization of the country should take into account the monitoring and control activities on the production, import and export of drugs in order to reduce the possibility of any drug fraud.
Suggestions
A. According to the results obtained in this study, it is
recommended to carry out similar studies at the level of broiler
chicken breeding flocks in other regions of Iran in order to
better determine the importance of subclinical coccidiosis and
the drug resistance of coccidiosis.
B. It is suggested to carry out detailed studies in order to
accurately determine the resistant species with molecular
methods, and it is recommended to prepare and identify drugs
sensitive to these resistant species.
References
- Conway DP, McKenzie ME (2007) Poultry Coccidiosis: Diagnostic and Testing Procedures. 3rd (edn), Blackwell Ltd Publishers, USA, pp. 41-62.
- Williams R (2002) Anticoccidial vaccines for broiler chickens: Pathways to success. Avian Pathology 31(4): 317-53.
- Razmi GR, Kalideri AG (2000) Prevalence of subclinical coccidiosis in broiler-chicken farms in the municipality of Mashhad, Khorasan, Iran. Preventive Veterinary Medicine 44(3-4): 247-253.
- Trees AJ (2008) Parasitic diseases. In: Saunders WB (Ed.), 6th (edn), Poultry Diseases, pp. 444-67.
- Calneck BW, Reid WM (1997) Disease of poultry. 10th (edn), Lowa State University Press, USA, pp. 856-83.
- Jordan FTW (1996) Poultry Disease. 4th (edn), Bailliere Tindal Publishers, USA, pp. 261-276.
- Gharekhani J, Dehkordi ZS, Bahrami M (2014) Prevalence of coccidiosis in broiler chicken farms in western Iran. Journal of Veterinary Medicine 2014: 980604.
- Andrabi SM, Ahmad MM, Shahab M (1998) Furazolidone treatment suppresses pubertal testosterone secretion in male broiler breeder birds (Gallus domesticus). Vet Hum Toxicol 40(6): 321-325.
- Nematollahi A, Moghaddam G, Pourabad RF (2009) Prevalence of Eimeria species among broiler chicks in Tabriz (Northwest of Iran). Mun Ent Zool 4(1): 53-58.
- Anadón A, Larrañaga MRM (2014) Veterinary drugs residues: Coccidiostats A2 - Motarjemi, Yasmine. Encyclopedia of Food Safety. Waltham: Academic Press, USA, pp. 63-75.
- Martello R (1990) Detection of subclinical coccidiosis. World Poultry, pp. 82-83.
- (1989) In: Salisch H (Ed.), Control of subclinical coccidiosis: Selection of chickens for post mortem examinations. Proceeding of 5th Inernational Coccidiosis Conference.
- Braunius WW (1983) Epidemiology of Eimeria in broiler flocks and the effect of anticoccidial drugs on the economic performance. Avian pathology 12(1): 23-33.
- (1982) In: Hamet W (Ed.), Epidemiology study on coccidiosis in broiler fowl. Bulletin de L'Acodemic Veterinaire de France, France.
- Henken AM, Goelema JO, Neijenhuis F, Vertommen MH, Bos VDJ, et al. (1992) Multivariate epidemiological approach to coccidiosis in broilers. Poultry science 71(11): 1849-1856.
- Voeten AC, Orthel FW, Rijen MAV (1988) Analysis of losses due to subclinical small intestinal coccidiosis caused by Eimeria acervulina and Eimeria maxima under field conditions. Tijdschr Diergeneeskd 113(18): 989-998.
- Swayne DE, Glisson JR, McDougald LR, Nolan LK, Suarez DL, et al. (2013) Diseases of Poultry. 13th (edn), Veterinary Medicine - Farm Animals, Wiley-Blackwell, pp. 1148-1166.
- Mattiello R (1990) Detect subclinical coccidiosis. Misset’s World Poultry Misset, pp. 82-83.
- Chapman HD (1984) Drug resistance in avian coccidia (A Review). Vet Parasitol 15(1): 11-27.
- Voeten V, Braunius W (1981) Subclinical coccidiosis in broilers. A comparative investigation of detection methods [Netherlands]. Archives of Poultry Science.
- Rogers EF, Clark RL, Becker HJ, Pessolano AA, Leanza WJ, et al. (1964) Anticoccidial Activity of 4-Amino-2-Ethoxy-benzoic Acid and Related Compounds. Proceedings of the Society for Experimental Biology and Medicine 117(2): 488-492.
- Watkins Kl, Bafundo KW, Donovan DJ (1990) Research note: Anticoccidial effect of monensin against Eimeria mitis and Eimeria dispersa. Poultry science 69(6): 1009-1011.
- Mathis G, Froyman R, Irion T, Kennedy T (2003) Coccidiosis control with toltrazuril in conjunction with anticoccidial medicated or nonmedicated feed. Avian diseases 47(2): 463-469.
- Rubin R, McLoughlin D, Costello L, Wehr E (1956) The efficacy of nicarbazin as a prophylactic drug in cecal coccidiosis of chickens. Poultry Science 35(4): 856-860.
- (2001) In: Mathis G, Lang M (Eds.), Comparative efficacy of Coccivac-B in broiler chickens. Proceeding of the 8th International Coccidiosis Conference.
- In: Challey J, Johnson C (Eds.), (1968) Penetration and development of eimeria maxima sporozoites and static effect of decoquinate. Poultry science.
- McLoughlin D, Gardiner JL (1967) Drug resistance in Eimeria tenella. V. The experimental development of a nicarbazin-resistant strain. The Journal of Parasitology 53(5): 930-932.
- McLoughlin D, Chute MB (1975) Sequential use of coccidiostats: effect on development by Eimeria tenella of resistance to amprolium, nicarbazin, Unistat, and zoalene. Avian diseases 19(3): 424-428.
- Tamas T, Schleim K, Wilks G (1991) Development of resistance against amprolium, nicarbazin, maduramicin, lasalocid and Maxiban. Poult Sci 70(Suppl 1): 1-119.
- Jeffers T (1974) Anticoccidial drug resistance: Differences between Eimeria acervulina and tenella strains within broiler houses. Poultry science 53(3): 1009-1013.
- Jeffers T, Challey J (1973) Collateral sensitivity to 4-hydroxyquinolines in Eimeria acervulina strains resistant to meticlorpindol. The Journal of parasitology 59(4): 624-30.
- Jeffers T K (1974) Eimeria acervulina and E. maxima: Incidence and anticoccidial drug resistance of isolants in major broiler-producing areas. Avian Dis 18(3): 331-342.
- Yadav A, Gupta S K (2001) Study of resistance against some ionophores in Eimeria tenella field isolates. Veterinary Parasitology 102(1-2): 69-75.
- Arabkhazaeli F, Modrisanei M, Nabian S, Mansoori B, Madani A ( 2013) Evaluating the resistance of eimeria spp. Field isolates to anticoccidial drugs using three different indices. Iran J Parasitol 8(2):234-41.
- Chapman HD (2014) Milestones in avian coccidiosis research: A review. Poultry science 93(3): 501-511.
- Keppens L, Groote Gd (1980) Study of the value of salinomycin as a coccidiostat for broiler chickens. Agricultural Review 37
- Keshavarz K, McDougald LR (1981) Influence of anticoccidial drugs on losses of broiler chickens from heat stress and coccidiosis. Poul Sci 60(11): 2423-2428.
- Montemayor D, Casas J, Moreno R, (1990) Anticoccidial efficacy of (R-64433) diclazuril in broiler chickens (battery trial and floor pen test with three species of coccidia in Mexico). Proceedings-Western Poultry Disease Conference (USA)
- Mitani M, Yamanishi T, Miyazaki Y, Ōtake N (1976) Salinomycin effects on mitochondrial ion translocation and respiration. Antimicrobial agents and chemotherapy 9(4): 655-660.
- Mitrovic M, Bauernfeind J. Sulfadimethoxine* (1967) Therapy of Avian Coccidiosis. Poultry science 46(2): 402-11.
- Chapman H, Hacker A (1993) The effects of shuttle programs upon the growth of broilers and the development of immunity to Eimeria species. Poultry science 72(4): 658-663.
- Choquet D, Felsenfeld D P, Sheetz M P (1997) Extracellular matrix rigidity causes strengthening of integrin-cytoskeleton linkages. Cell 88(1): 39-48.
- Djemai S, Mekroud A, Jenkins MC (2016) Evaluation of ionophore sensitivity of Eimeria acervulina and Eimeria maxima isolated from the Algerian to Jijel province poultry farms. Veterinary Parasitology 224: 77-81.
- Daugschies A, Gässlein U, Rommel M (1998) Comparative efficacy of anticoccidials under the conditions of commercial broiler production and in battery trials. Vet Parasitol 76(3): 163-171.
- Chapman H (1986) Isolates of Eimeria tenella: Studies on resistance to ionophorous anticoccidial drugs. Research in Veterinary Science 41(2): 281-282.
- Joyner L, Davies S, Kendall S (1963) Chemotherapy of coccidiosis. Experimental chemotherapy 1: 445-80.
- Peek H, Landman W (2003) Resistance to anticoccidial drugs of Dutch avian Eimeria spp. field isolates originating from 1996, 1999 and 2001. Avian Pathology 32(4): 391-401.
- Lux R (1954) The chemotherapy of Eimeria tenella. I. Diaminopyrimidines and dihydrotriazenes. Antibiotics & chemotherapy (Northfield, Ill) 4(9): 971-977.
- MacPherson I (1978) Coccidiosis in broilers and Turkeys. Avian Coccidiosis, p. 465.
- Vertommen M, Peek H, Van der Laan A (1990) Efficacy of toltrazuril in broilers and development of a laboratory model for sensitivity testing of Eimeria field isolates. Veterinary Quarterly 12(3): 183-192.
- McDougald L, Roberson E (1988) Antiprotozoan drugs.
- Kawazoe U, Di Fabio J (1994) Resistance to diclazuril in field isolates of Eimeria species obtained from commercial broiler flocks in Brazil. Avian pathology 23(2): 305-311.
© 2023 Arash Ramezanpour. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






