- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Effects of Injectable Mineral and Vitamin Supplementation on the Fertility of Dairy Cows in FTAI Protocol
Márcio Luiz Denck Tramontin1, Carlos Renato de Freitas Guaitolini2, André Maciel Crespilho3, Francislaine Aparecida dos Reis Livero1, Danielle Andressa Oliveira Sestari1, Alecsandro Silva de Lima Elias1, Alyson Liberali Araujo1, and Rosiara Rosária Dias Maziero2*
1Universidade Paranaense, Umuarama, Paraná, Brazil
2CRV, Brazil
3Universidade Santo Amaro, São Paulo, São Paulo, Brazil/p>
*Corresponding author: Rosiara Rosária Dias Maziero, Department of Animal Reproduction and Veterinary Radiology, CRV, Brazil
Submission: December 02, 2022;Published: December 22, 2022

ISSN: 2576-9162 Volume9 Issue3
Abstract
This study aimed to verify the possible interaction between injectable mineral and vitamin supplementation (Kit Adaptador® MIN e Adaptador® VIT, Biogénesis Bagó, Brazil) performed at the beginning of fixed-time artificial insemination (FTAI) protocol on reproductive performance and Holstein cows’ fertility. For the study, 100 animals were selected, divided into a non-supplemented control group (CG, n=50) and a treated group (TG, n=50), in which mineral and vitamin supplementation (1mL/100 kg live weight, IM) on the first day of the FTAI protocol (D0) based on intravaginal progesterone (P4) implant and estradiol benzoate. All cows were inseminated ten days after the beginning of the protocol (D10), with blood samples taken at D0 and D17 to quantify the activity of the superoxide dismutase enzyme (SOD) and to measure P4. Ovarian and uterine ultrasound assessments were conducted at moments D0 and D17, and a new exam was performed 30 days after FTAI for pregnancy diagnosis. A larger diameter of the corpus luteum was observed (CG=2.14±0.11cm vs TG=2.64±0.11cm, P=0.0037) and SOD activity (CG=22% vs TG=25.36%, P=0.045) for cows that received mineral and vitamin supplementation. Additionally, differences were observed for the pregnancy rate when comparing the different groups (CG=18.18% vs TG=39.13%, P=0.0029). It is concluded that the mineral and vitamin supplementation performed at the beginning of the FTAI protocol can influence the formation and development of the postovulation corpus luteum, increase the pregnancy rate and stimulating important endogenous antioxidant mechanisms of Holstein cows.
Keywords:Antioxidants; FTAI; Minerals; Pregnancy; Vitamins; Intravaginal progesterone
Introduction
High-yielding dairy cows require a diet that provides all the necessary nutrients for proper milk production. Carbohydrates, amino acids, fatty acids, minerals, vitamins, and water are required to meet the demand of the mammary gland during the lactation period, which are also responsible for maintaining reproductive activity [1]. In detriment to the countless technological advances and new nutritional strategies implemented over the last decades for milk production, the fertility of dairy cows has been suffering a progressive and significant reduction over the last years resulting in a fall in animal reproductive and productive performance [2]. Although the drop in the conception rates for dairy cows has a multifactorial origin [3], the morphofunctional assessment of the corpus luteum [4] and the quantification of serum progesterone levels have proved to be valuable tools for the understanding of animal fertility variations [5,6]. In this context, previous studies have shown that cows with lower levels of plasma progesterone (P4) show less growth of ovulatory follicles, associated with greater embryonic loss [7] and a drop in the conception rates [8].
Functional regulation of CL depends, among other factors, on correct nutritional management. According to Kamada [5], selenium supplementation in the postpartum period is able to increase the production of P4 by CL. Likewise, previous studies have shown that dietary fat supplementation is also effective for increasing plasma P4 concentrations [9-11]. Vitamins and vitamin precursors such as beta-carotene may also favor the restoration of postpartum luteal activity in dairy cows [12], being able to stimulate follicular growth and P4 synthesis [13]. Thus, from the correct nutritional, mineral, and vitamin supplementation, important biochemical pathways related to energy production are stimulated, which in turn imply not only an increase in the activity of the corpus luteum but also an inhibition of prostaglandin synthesis by acid linoleic derived from fat [14]. An increase in the metabolic demand associated with pregnancy, labor, and the start of lactation is expected for high milk production cows, events that also increase the production of oxygen free radicals [15]. Thus, the antioxidative activity of several components of the diet and its relationship with reproductive function has been discussed by several authors [16,17]. Arianmanesh et al. [18] related that in the presence of a mature CL there is a significant increase in oxidative stress, an event that has a positive interaction with increases in progesterone levels. In addition, previous studies haveassociated an increase in the production of free radicals and oxidative stress to the pathogenesis of several infertility manifestations in women and domestic females [16]. In this context, several antioxidant enzymes such as superoxide dismutase, paraoxonase, and glutathione peroxidase, in addition to pro-inflammatory cytokines such as Interleukin-6, can be beneficial for reproductive activity, improving the rate of implantation and maintenance of pregnancy, degrading free radicals of oxygen that have deleterious effects on the organism [19-21] (Smith et al.,1998; Bedaiwy et al., 2003; Oyawoye et al., 2003). This study aimed to evaluate the effect of a single dose of injectable mineral and vitamin supplement on the reproductive parameters and fertility of Holstein cows submitted to estrus synchronization for artificial insemination, to test whether the hypothesis that strategic supplementation of cows at the beginning of the FTAI protocol can improve the reproductive performance of bovine females.
Marerial and Methods
Animals and experimental groups
The experiment was approved by the Research Ethics Committee Involving Animal Experimentation at Universidade Paranaense - (UNIPAR), under protocol number 35552/2019, on August 23, 2018. For the study, 100 cyclical Holstein cows, PO, were selected, all in lactation (±150 days, on average), pluriparous, with body condition scores between 3 and 3.5 (scale of 1-5), after a period waiting for 60 days. All cows were subjected to weighing and body condition score assessment at the beginning of the experiment (D0). The animals were randomly divided into two groups: Control Group (CG; n=50, administration of 1mL/100 Kg of live weight of sterile 0.9% saline, IM) and Treated Group (TG; n=50) who received a single dose of mineral and vitamin supplement (Kit Adaptador® MIN1 e Adaptador® VIT2 - Biogénesis Bagó, respectively, at a dose of 1mL/100kg live weight, IM). The composition of the product (Kit Adaptador®) is: 5,950,000 IU of vitamin A; 5000 IU of vitamin E, 1g of copper edetate, 4g of zinc edetate, 1g of manganese edetate and 0.5g of selenium in the form of sodium selenite for every 100mL of excipients q.s.p. All applications were performed on the first day of the FTAI protocol (D0).
For the synchronization of the estrous cycle, a protocol based on an intravaginal implant containing 1g of progesterone (Dispositivo Cronipres monodose®; Biogénesis Bag, Curitiba, Paraná, Brazil) and a single dose of 2mg of estradiol benzoate (Bioestrogen®; IM; Biogénesis Bagó, Curitiba, Paraná, Brazil) on day zero (D0). After 8 days (D8), the progesterone implant was removed and 150μg of sodium cloprostenol was administered (Croniben®; IM; Biogénesis Bagó, Curitiba, Paraná, Brazil), 400 I.U. eCG (Ecegon®; IM; Biogénesis Bagó, Curitiba, Paraná, Brazil), 0.5mg of estradiol cypionate (Croni- Cip®; IM; Biogénesis Bagó, Curitiba, Paraná, Brazil) and application of paint at the base of the tail of each cow using marker sticks to assist in the detection of heat. Fixed-time artificial insemination was performed 48 hours after the removal of the progesterone implant (D10) in all females that manifested heat (they lost the ink mark at the base of the tail, according to Madureira et al. [19]. For animals that did not show heat at the time of FTAI (still marked by stick ink), 10.5mcg of buserelin acetate (Gonaxal®; IM; Biogénesis Bagó, Curitiba, Paraná, Brazil) were administered to perform FTAI 6 hours later
Endocrine and biochemical assessments
Venous blood samples were collected from all animals by jugular venipuncture on the day the hormonal protocol started (D0) and 7 days after FTAI (D17) was performed to assess the activity of the superoxide dismutase enzyme (SOD) and for the measurement of plasma progesterone.
Analysis of superoxide dismutase activity: To investigate the activity of the Superoxide Dismutase Enzyme (SOD), the pyrogallol oxidation method was used, a compound that undergoes oxidation from pH variations, according to the technique described by Gao et al. For this purpose, 60μL of serum were diluted in 1327.5μL of Tris- EDTA buffer (0.4 M, pH 8), vortexed, and mixed in 75μL of pyrogallol solution (15mM). The reaction was incubated for 30min at room temperature and blocked by the addition of 37.5μL of hydrochloric acid (1M). The resulting absorbance of the supernatant was measured on a benchtop spectrophotometer using a 405nm filter. The enzymatic activity was expressed in U of SOD/mg of protein.
Measurement of plasma progesterone: To quantify serum progesterone levels, an ELISA kit (Progesterone AccuBind®, MonoBind Inc, Lake Forest, CA) was used, according to the methodology described by Kunbar et al. [20]. The test results were obtained through a benchtop spectrophotometer from the absorbance reading of each well of the ELISA plate at 450nm, using a reference wavelength of 620-630nm, according to the manufacturer’s recommendations. The accuracy of the assay was monitored by calculating the intra-assay coefficient of variation (4.1%), the results being expressed in ng/ml.
Reproductive assessments
All animals were submitted to palpation and transrectal ultrasound (Sonoscape A5v, probe 5-16MHz) for ovarian and uterine evaluation on the first day of the hormonal protocol (D0) and, subsequently, on the day of FTAI (D10) and D17. In the clinical reproductive examination, the following variables were considered: ovarian diameter (cm), ovarian area (cm), and uterine diameter (cm) at D0; presence and diameter of the dominant follicle (cm) at D10; and corpus luteum diameter (cm) in D17. The pregnancy diagnosis was made by ultrasound, 30 days after FTAI.
Statistical analysis
For the statistical analysis, the software Statistical Analysis System (SAS® Institute Inc., 2001) was used. Initially, the Shapiro- Wilk test (Proc Univariate) was used to test the normality of residues and the Chi-square test for homogeneity. Using a variance analysis model (Proc GLM), the main effect of the treatment, in addition to its possible interactions, on the ovarian and uterine size and diameter, follicular and corpus luteum size, weight, and body condition score was evaluated, plasma progesterone concentration and SOD activity. The averages generated were adjusted using the LSMEANS command, prior to statistical analysis. To create the graphics, the computer software Graphpad Prism® version 5.0 was used. All results were presented as mean ± standard error of the mean. Differences were considered when P<0.05.
Results
No differences were observed when comparing the different groups for the diameter and ovarian area, or for the diameter of the body of the uterus in the reproductive evaluation conducted at D0. The average diameter of the corpus luteum, which was higher for the group that received mineral and vitamin supplementation (P=0.0037), no differences were observed for any of the morphometric parameters studied when comparing the two experimental groups on the day of FTAI (Table 1). The conception rate at FTAI did differ between groups (CG=18.18±0.09%; TG=39.13±0.09%; P=0.0029). Likewise, weight (CG=606.43±16.48 and TG=622.87±15.92, P=0.4779) and body condition score (CG=3.04±0.14 and TG=3.19±0.14, P=0.4678) did not differ between groups. Although the activity of the superoxide dismutase enzyme did not change when the different groups were compared at D0 (CG=113.80±2.47; TG=104.10±1.84) and D17 (CG=138.90±3.48; TG=130.50±2.87), a higher mean elevation (difference between the SOD activity at D17 in relation to the enzymatic activity shown on the first day of the FTAI protocol) was observed in the SOD activity for cows that received vitamin and mineral supplementation (Figure 1).
Figure 1:Relationship between the initial (D0) and final (D17) serum activity of the superoxide dismutase enzyme comparing cows in the control group or that received mineral and vitamin supplementation (Kit Adaptador® MIN e Adaptador® VIT, IM; Biogénesis Bagó, Brazil) on the first day of the FTAI protocol.
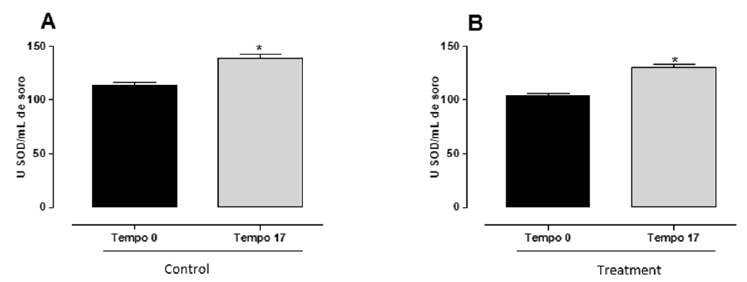
Table 1: Reproductive parameters evaluated on the day of artificial insemination (D10) for cows in the control groups (CG) or that will receive mineral and vitamin supplementation (TG) on the first day of the FTAI protocol.
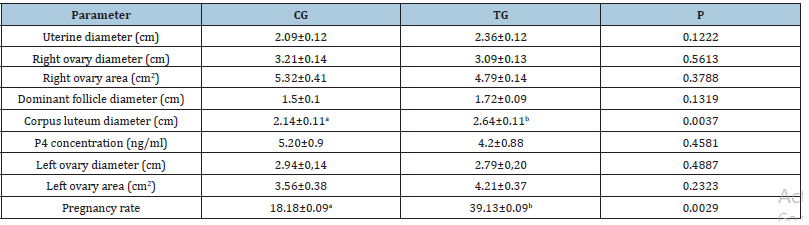
a,bDifferent lower-case letters on the same line indicate the statistical differences found (P<0.05).
Discussion
Strategic vitamin and mineral supplementation during the pre-FTAI period have been associated with improved reproductive performance in beef cows [21]. Previous studies have reported that the lack of selenium, zinc, and copper occurs in most animals, throughout the national territory, especially during periods of greater metabolic demand such as growth and lactation [22]. In the present research carried out with Holstein cows, it was found that the animals in the treated group, which received an intramuscular injection of vitamins and minerals, had a larger diameter of the corpus luteum, compared to the control group (C=2.14±0.11 cm vs T=2.64±0.11 cm) evaluated 7 days after FTAI (P<0.05). Regarding plasma progesterone concentrations, these did not differ (P=0.4581) between groups, which corroborates with Leal et al. who found no significant differences in the pregnancy rate in relation to the size/diameter of the CL (small, medium, and large). CLs considered large produce more progesterone than small and medium CLs, but this did not affect pregnancy rates. These data, however, differ from those presented by Velho et al. [23] and Gonella-Diaza et al. [24] observed a correlation between the area of the corpus luteum and the plasma concentrations of progesterone in pregnancy rates. Luteal bodies with an upper area determined a higher plasma P4 concentration and, therefore, a higher pregnancy rate. For the diameter parameter of dominant follicles, there was no difference between groups (P>0.05). These data are different from those reported by Santin et al. [21] in beef cows, which had a larger follicle diameter in the treated group (C=1.31cm vs T=1.48cm) with mineral and vitamin supplementation.
In this study, the animals’ antioxidative status was assessed by measuring the activity of the superoxide dismutase enzyme (SOD), showing that when comparing the groups on days 0 and 17 of the protocol, an increase of 22.05% in the activity of the enzyme was observed in the control group and an increase of 25.36% in the treated group (P = 0.045). In the work presented by Maturana Filho et al. [22], in which 476 Nellore animals were used for the control group (NaCl 0.8%) and 756 animals in the treated group (KIT Adaptador® MIN e Adaptador® VIT; Biogénesis Bagó), it was found that the enzyme activity superoxide dismutase in FTAI was higher in the treated group (C=1095U/G Hb vs T=2126U/G Hb). The superoxide dismutase enzyme is synthesized by luteal cells, which reinforces the survival of the corpus luteum by eliminating the superoxide radicals, toxic agents of regression of luteal structures, by inhibiting the activity of P450 aromatase. Nitric oxide is a potent vasodilator, originating from L-arginine through the action of nitric oxide synthase (NOS) [25-30]. In the bovine corpus luteum, there are two types of NOS: endothelial NO (eNO) and inducible NO (iNO). In bovine CL, NOS is expressed in greater quantity during the final luteal phase, which is related to the increase in blood flow. NO can be an important mediator of luteolysis in cows, as it directly inhibits progesterone secretion and induces apoptosis in bovine luteal cells. The CL in the middle, final, and regression phases also has several positive areas for eNOS [31].
During the middle luteal phase, PGF2α stimulates mRNA expression for eNOS and iNOS in the peripheral area of the CL, while in the central area this effect is not observed; such a phenomenon indicates that the different acute actions of PGF2α depend not only on the luteal phase but also on the CL region [32,33]. The increase in peripheral blood flow is one of the first physiological indicators of the action of NO in response to PGF2α. However, NO acts not only in functional luteolysis, but also participates in the structural regression of CL, modifying the expression of proteins involved with apoptosis [33,34]. Nitric oxide also regulates the concentration of the antioxidant enzyme superoxide dismutase (SOD) in luteal endothelial cells, depending on the time of exposure. Initially, there is an increase in SOD; this increase probably represents a cellular protective response of CL against oxidative stress induced by PGF2α during functional luteolysis. However, NO induces a reduction in SOD, which, in turn, results in an increase in Reactive Oxygen Species (ROS), a phenomenon that is important for structural luteolysis [35]. In this study, it is clear that the SOD increased in the analysis at D17, that is, 7 days after the AI. Furthermore, in this context, Aoki et al. [31] showed that oxidative damage can be assessed by analyzing the concentrations of Thiobarbituric Acid Reactive Substances (TBARS). In this study, they observed that in the supplemented group (2.9nm/mg protein), the concentrations of Thiobarbituric Acid Reactive Substances remained and increased in the control group (2.8 to 3.39nm/mg protein) (P<0.05). The use of TBARS is based on the reaction of malondialdehyde with Thiobarbituric Acid, which measures lipid peroxidation.
Regarding the pregnancy data obtained in the present study, it was found that there was difference between the groups (C=18.18±0.09% vs T=39.13±0.09%). This experiment is similar of the work presented by Lisarraga et al. [36], in which pregnancy rates were 54.2% for the treated group and 46.7% for the control group, in Hereford cows, in the Buenos Aires region, Argentina. For this, homogeneous groups were formed at random, separated into a treated group, where 5mL of mineral and injectable vitamin supplementation (Kit Adaptador®; Biogénesis Bagó) were used, subcutaneously at the time of placement of intravaginal progesterone devices and another group control. In another study by Santin et al. [21], there was an increase in the pregnancy rate when injectable mineral and vitamin supplementation (KIT Adaptador® MIN e Adaptador® VIT, Biogénesis Bagó), in Nellore cows (n=408). The conception rate at 30 days was higher in the cows that received the KIT Adaptador® (G1=52.5%, G2=61%, G3=57.7% and G4=51.5%), as well as the pregnancy rate at 60 days (P<0.05) was higher in the treated animals (G1=51.4%, G2=60%, G3=55.7% and G4=49.5%). Similar to our study, Muanis [32] found that there was an effect of vitamin and mineral supplementation (Adapter Kit) on the conception rate (Control=45.1%; and Treated=49.8%) and pregnancy rate (Control=35.1%; and Treated=37.8%) of cows and heifers (P<0.05) receiving Nelore embryos. The injectable mineral and injectable mineral and vitamin supplementation in embryo recipients had a beneficial effect on the fertility of embryo recipients with low body condition status. Different from our results, Simonetti [33] found no difference in pregnancy data when using the Kit Adaptador® MIN and Adaptador® VIT, via IM. This author observed that in animals without the use of mineral and vitamin supplementation the pregnancy rate was 56.2% (36/64) and in the treated group it was 52.3% (44/84).
Unzué et al. [34] carried out a study using heifers aged 15 months and the results were variable. When performing 3 repetitions of mineral and vitamin supplementation, they observed positive effects on pregnancy data in only one of them. Additionally, Garcia Eyherabide et al. [35] found different results when using heifers, there were no differences in pregnancy rates in supplemented animals, but when administering the Kit Adaptador® MIN and Adaptador® VIT, via IM, in cows in the second service, there was an increase in the pregnancy rate. In addition, Fazzio et al. [36] found a positive effect of Kit Adaptador® MIN and Adaptador® VIT, via IM, on pregnancy rates, with 59.6% in the treated group and 48.2% in the control group (P<0.05).
Conclusion
With this study, it was concluded that there is an interference from the use of mineral and vitamin supplementation in the functionality of the corpus luteum and pregnancy rates. The use of injectable mineral and vitamin supplementation is an excellent option for producers and technicians as an alternative to supply occasional needs, in which occurs oxidative stress in animals. It is an extremely efficient and low-cost strategy, which it is an extremely efficient and low-cost strategy, which aims to improve artificial insemination programs in fixed time of Holstein cows.
References
- Erickson PS, Kalscheurs KF (2020) Nutrition and feeding of dairy cattle. Anim Agric 157-180.
- Portillo BA, Pollott GE (2013) The relationship between fertility and lactation characteristics in Holstein cows on United Kington commercial dairy farms. J Dairy Sci 96(1): 635-646.
- Skovorodin E, Bogolyuk S, Bazekin G, Sharipov A, Khoknlov R (2020) Morphology and histochemistry of the corpus luteum (CL) of ovaries of pregnant and infertile cows. Am J Anim Vet Sci 15(4): 257-265.
- Varughese EE, Brar PS, Ghuman SS (2017) Vascularization to preovulatory follicle and corpus luteum-a valuable predictor of fertility in dairy cows. Theriogenology 103: 59-68.
- Kamada H (2017) Effects of selenium-rich yeast supplementation on the plasma progesterone levels of postpartum dairy cows. Asian-Australas J Anim Sci 30(3): 347-354.
- Tarekegn GM, Gullstrand P, Strandberg E, Báge R, Rius-Vilarrasa E, et al. (2019) Genetic parameters of endocrine fertility traits based on in-line milk progesterone profiles in Swedish Red and Holstein dairy cows. J Dairy Sci 102(12): 11207-11216.
- Martins JPN, Wang D, Mu N, Rossi GF, Martini AP, et al. (2018) Level of circulating concentrations of progesterone during ovulatory follicle development affects timing of pregnancy loss in lactating dairy cows. J Dairy Sci 101(11): 10505-10525.
- Inskeep EK (2004) Preovulatory and post maternal recognition effects of concentrations of progesterone on embryonic survival in the cow. J Anim Sci 82: E24-E39.
- Xu W, Vervoort J, Saccent E, Kemp B, Hoeij JV, et al. (2020) Relationship between energy balance and metabolic profiles in plasma and milk of dairy cows in early lactation. J Dairy Sci 103(5): 4795-4805.
- Hutchinson A, Hennessy AA, Waters SM, Dewhurst RJ, Evans ACO, et al. (2012) Effect of supplementation with different fat sources on the mechanisms involved in reproductive performance in lactating dairy cattle. Theriogenology 78(1):12-27.
- Temesgen MY, Assen AA, Gizaw TT, Minalu BA, Mersha AY (2022) Factors affecting calving to conception internal (days open) in dairy cows located at Dessie and Kombolcha tows, Ethiopia. PLoS One 17(2): e0264029.
- Kawashima C, Nagashima S, Sawada K, Schweigert FJ, Miyamoto A, et al. (2010) Effect of β-carotene supply during close-up dry period on the onset of first postpartum luteal activity in dairy cows. Reprod Domest Anim 45(6): e282-e287.
- Rodriguez GA, Herrera CAM, Martinez RR, Tapia RD, Hallford DM, et al. (2009) Short-term intake of β-carotene-supplemented diets enhances ovarian function and progesterone synthesis in goats. J Anim Physiol Anim Nutr (Berl) 93(6): 710-715.
- Vanegas JA, Reynolds J, Atwill ER (2004) Effects of an injectable trace mineral supplement on first-service conception rate of dairy cows. J Dairy Sci 87(11): 3665-3671.
- Nazari A, Dirandeh E, Pirsaraei ZA, Deldar H (2019) Antioxidant levels, copper and zinc concentrations were associated with postpartum luteal activity, pregnancy loss and pregnancy status in Holstein dairy cows. Theriogenology 133: 97-193.
- Younis A, Clower C, Nelsen D, Butler W, Carvalho A, et al. (2012) The relationship between pregnancy and oxidative stress markers on patients undergoing ovarian stimulations. J Assist Reprod Genet 29(10): 1083-1089.
- Abuelo A, Hernández J, Benedito JL, Castilho C (2019) Redox biology in transition periods of dairy cattle: role in the health of periparturient and neonatal animals. Antioxidants (Basel) 8(1): 1-19.
- Arianmanesh M, Mclntosh RH, Lea RG, Fowler PA, Gubory KHA (2011) Ovine corpus luteum proteins, with functions including oxidative stress and lipid metabolism, show complex alterations during implantation. J Endocrinol 210(1): 47-58.
- Madureira AML, Burnett TA, Marques JCS, Moore AL, Borchardt S, et al. (2022) Occurrence and greater intensity of estrus in recipient lactating dairy cows improve pregnancy per embryo transfer. J Dairy Sci 105(1): 877-888.
- Kunbar HK, Memon AA, Kachiwal AB, Shaikh SA, Bukhari HS, et al. (2017) Plasma progesterone concentration during estrus cycle detected through ELIZA kit method in Kamohri goats. Sch J Agric Vet Sci 4(1): 1-5.
- Santin T, Filho MM, Lemes KM, Silva MA, Gonçalves RL, et al. (2016) Evaluation of different injectable mineral and vitamin supplementation strategies (Adaptor KIT® MIN and Adapter® VIT, Biogénesis Bagó) in improving pregnancy rates in beef cows. Brazilian Society of Embryo Technology, Brazil.
- Filho MM, Lemes KM, Silva MA, Santin T, Gonçalves RL, et al. (2016) Effect of injectable mineral and vitamin supplementation (Adaptor kit® MIN and Adapter® VIT, Biogénesis Bagó) on pregnancy rates in beef cows. Brazilian Society of Embryo Technology, Brazil.
- Velho GS, Rovani MT, Ferreira R, Gasperin BG, Dalto AGC (2022) Blood perfusion and diameter of bovine corpus luteum as predictors of luteal function in early pregnancy. Reprod Domest Anim 57(3): 246-252.
- Diaza AMG, Holguín G, Montana D, Valbuena D (2013) Corpus luteum diameter and embryo developmental stage are associated with pregnancy rate: data analysis from 17,521 embryo transfers from a commercial in vitro bovine embryo production program. Anim Reprod 10(2): 106-111.
- Mlyczyńska E, Kieżun M, Kurowska P, Agnieszka R, Mathilde D, et al. (2022) New aspects of corpus luteum regulation in physiological and pathological conditions: Involvement of adipokines and neuropeptides. Cells 11(6): 957.
- Trevisol E, Ferreira JCP, Ackermann CL, Destro FC, Amaral JB (2013) Luteoysis in cattle. Rev Bras Reprod Anim 37(1): 29-36.
- Shirasuna K, Watanabe S, Asaki T, Wijayagunawardane MPB, Sasahara K, et al. (2008) Prostaglandin F2α increase endothelial nitric oxide synthase in the periphery of the bovine corpus luteum: the possible regulation of blood flow at an early stage of luteolysis. Reproduction 135(4): 527-539.
- Shirasuna K, Asahi T, Sasaki M, Shimizu T, Miyamoto A (2010) Distribution of arteriolovenous vessels, capillaries and eNOS expression in the bovine corpus luteum during the estrous cycles: a possible implication of different sensitivity by luteal phase to PGF2α in the increase of luteal blood flow. J Reprod Dev 56(1): 124-130.
- Shirasuna K, Sasahara K, Matsui M, Shimizu T, Miyamoto A (2010) Prostaglandin F2α differentially affects mRNA expression relating to angiogenesis, vasoactivation, and prostaglandins in the early and mid-corpus luteum in the cow. J Reprod Dev 56(4): 428-436.
- Sugino N (2005) Reactive oxygen species in ovarian physiology. Reprod Med Biol 4(1): 31-44.
- Aoki M, Oshida T, Sakaguchi M (2008) The comparison of thiobarbituric acid reactive substances (TBARS) concentrations in plasma and serum from dairy cattle. J Vet Med Sci 70(1): 107-110.
- Muanis GCMN (2020) Injectable mineral and vitamin supplementation (Kit Adaptador® Min e Vit, Biogénesis Bagó) in the fertility of bovine embryo recipients. Dissertation, Viçosa, Brazil.
- Simonetti M, Mihura H, Cabodevilla J, Callejas S (2018) Effect of vitamin and mineral supplementation on the percentage of pregnancy in cows inseminated at a fixed time.
- Unzué TL, Chayer R, Chaves SG, Cabodevila J, Callejas S (2019) Effect of a vitamin-mineral complex on pregnancy after fixed-time artificial insemination. Rev Inv Vet Peru 30(4): 1811-1815.
- Eyherabide LG, Muriel JC, Persico JMR (2017) Effect of the injectable supplementation of an antioxidant combination on the pregnancy rate in TAI protocols. En: XII International Symposium on Animal Reproduction. Cordoba, Argentina.
- Fazzio LE, Galvan WR, Pesoa JM, Pérsico JMR, Mattioli GA (2017) Injectable supplementation with vitamins and minerals with an antioxidant effect on the pregnancy rate of vaquillonas. XII International Symposium on Animal Reproduction. Cordoba, Argentina.
© 2022 Rosiara Rosária Dias Maziero. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






