- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Seroprevalence of Toxoplasma Gondii and Associated Risk Factors in Dairy Cows in Greece
Menelaos Lefkaditis1, Zoi Athanasakopoulou1, Marina Sofia1, RustemMpairamoglou2, Charalambos Billinis 1,3*
1Laboratory of Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Thessaly, Greece
2Veterinary clinic, Prefecture of Xanthi, Greece
3Faculty of Public and One Health, University of Thessaly, Greece Introduction
*Corresponding author: Charalambos Billinis, Laboratory of Microbiology and Parasitology, Faculty of Veterinary Medicine, University of Thessaly, Greece
Submission: October 27, 2022;Published: October 31, 2022

ISSN: 2576-9162 Volume9 Issue2
Abstract
Toxoplasmosis is a worldwide protozoosis which can affect all warm blooded animals, birds and humans, while cats and other Felidae are the only definitive hosts of this parasite. Dairy cows become infected orally, through ingestion of feed or water contaminated with T. gondii sporulated oocysts, but also through accidental ingestion of tissue cysts from infected intermediate hosts. In cows the infection is usually asymptomatic or causes mild symptoms. Natural cases of clinical toxoplasmosis in cows are usually manifested only as abortions. This study included 428 Holstein-Friesian dairy cows from 8 small farms located in Northern Greece, which had previously reported reproductive problems. A blood sample was collected from each cow and examined by indirect immunofluorescence (IFAT) for toxoplasmosis. The results revealed an overall prevalence of 28 (6.54%) positive and 52 (12.15%) suspect cows for the parasitosis. Among positive and suspect cows 64.28% (18/28) and 30.76% (16/52), respectively, had previous records of reproductive problems. Some reproductive parameters, such as the average number of lactations per cow and the average days after birth that animals were removed from farms due to infertility were 1.75 and 55.1 in positive cows and 2.77 and 561.5, respectively, in suspect cows. This study suggests that toxoplasmosis should be included in the differential diagnosis in cow farms where reproductive problems occur in order to reduce the economic losses of farmers. It also highlights the zoonotic importance of this parasitosis as well as the need for better preventative measures.
Keywords:Toxoplasma gondii; Dairy cattle; Zoonoses; Reproduction; Farm
Introduction
Toxoplasmosis is a zoonotic disease caused by the intracellular obligate apicomplexan protozoan parasite Toxoplasma gondii (Sarcocystidae), which is distributed throughout the world [1-4]. It is mainly transmitted to the intermediate hosts through the ingestion of oocysts shed by infected cats (or other Felidae). Cats are the only definitive host of the parasite and the only species where the sexual part of T. gondii life cycle can be completed [1,5,6] resulting in the release of the oocysts into the environment through the cat’s feces [5]. Cats can discharge those oocysts in the environment for a short period of time, only about 3 weeks throughout their life. After 1 to 3 days in the environment, oocyst sporulate and have the ability to infect a large variety of warm-blooded intermediate hosts of this parasite, such as domestic and wild animals, livestock, small rodents, birds, and humans [5,7,8]. Cats (domestic and wild) present with significantly high prevalence of toxoplasmosis in some cities of countries neighboring to Greece; 66.2% in the city of Ankara, Turkey [9], 42.3% in Perugia, Italy [10], 62.3% in Tirana, Albania [11], 29.3% in Milan, Italy [12] and 34.2% in Izmir, Turkey [13]. Dairy cows become infected mainly through ingestion of feed or water contaminated with T. gondii sporulated oocysts [14,15]. Many experimental infections in cows used oocysts, as the means of infection demonstrating that cows are susceptible to infection by this life stage of the parasite [14-17]. Although cows are herbivores, infections could also happen through the accidental ingestion of tissue cysts from infected intermediate hosts such as small rodents via contaminated food. Finally, transplacental infection can also take place and lead to infected fetuses [5].
In cows the infection is usually asymptomatic or causes mild symptoms [5]. Natural cases of clinical toxoplasmosis in cows is seldom manifested and only with abortions. Their confirmation can be performed with the isolation of T. gondii from the tissues of aborted fetuses [18]. In many areas of the world humans consume bovine meat. Therefore, consumption of cows’ meat may represent a risk for transmission of the disease to humans via the consumption of raw or undercooked meat [19,20]. The IFAT test is a simple and widely used method for the diagnosis of toxoplasmosis. This assay is based on the specific antigen-antibody interaction from diluted serum specimens with killed Toxoplasma tachyzoites. Fluorescentlabeled antibodies are commercially available for a variety of animal species, the method is safe and relatively inexpensive and kits are also commercially available [21,22]. The aim of this study is to emphasize that toxoplasmosis should never be excluded from the differential diagnosis in cow farms where reproductive problems exist. In the light of economic losses caused for farmers and most importantly of the zoonotic consequences of toxoplasmosis, it is imperative to establish preventative measures.
Marerial and Methods
This study included 428 Holstein-Friesian dairy cows from small farms located in northern Greece (prefecture of Xanthi) that had previously reported reproductive problems and which were sampled during 2021-2022. The cows originated from eight farms of which the smallest comprised of 30 and the largest of 150 cows. In all farms, dogs and cats coexisted with cows and no preventive antiparasitic treatment was given to any of the animals. A blood sample was collected from all the cows of each farm. All samples were examined by the method of indirect Immunofluorescence (IFAT) for toxoplasmosis [23]. According to this technique killed T. gondii tachyzoites are incubated with test serum, the fluorescent anti-species antibodies are added, and the results are read under a fluorescence microscope. All samples that were positive in the dilution 1/200 were marked as positive, while those samples that were positive in the dilution 1/100 were reported as suspect for T. gondii antibodies [24]. Various parameters were recorded during sampling. For the statistical analysis of the results Chi square test was used (factor of significance P> 0.005).
Results
Out of the 428 dairy cows tested for toxoplasmosis, 28 (6.54%) were found positive and 52 (12.15%) suspect of T. gondii antibodies. Positive and suspect cows were found in all the eight farms included in the study. The study also recorded a statistically significant higher frequency (P> 0.005) of positive cows manifesting infertility problems compared with suspect cows. In particular, 18 out of 28 positive cows (64.28%) presented infertility problems, while only 16 out of 52 suspect cows (30,76%) manifested such problems. The average number of lactation periods when the cows were removed from the farm due to infertility problems was statistically smaller in positive (1, 75) compared to suspect cows (2, 77) (Table 1). Moreover, the study showed that infertility problems were the main reason of removal of the animals from the farms. Various other parameters of reproductive problems and the removal of positive and suspect cows Tables 2 were also determined.
Table 1: Parameters recorded during sampling and their outcome for positive and suspect animals.
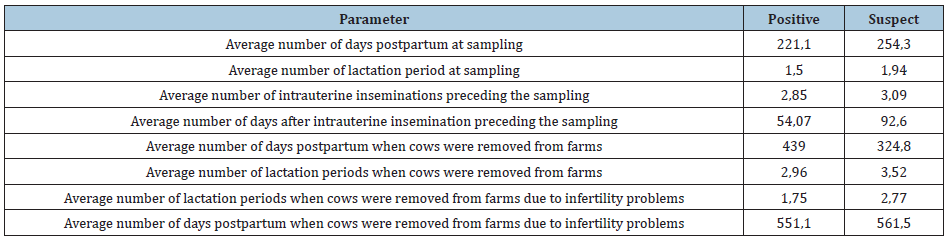
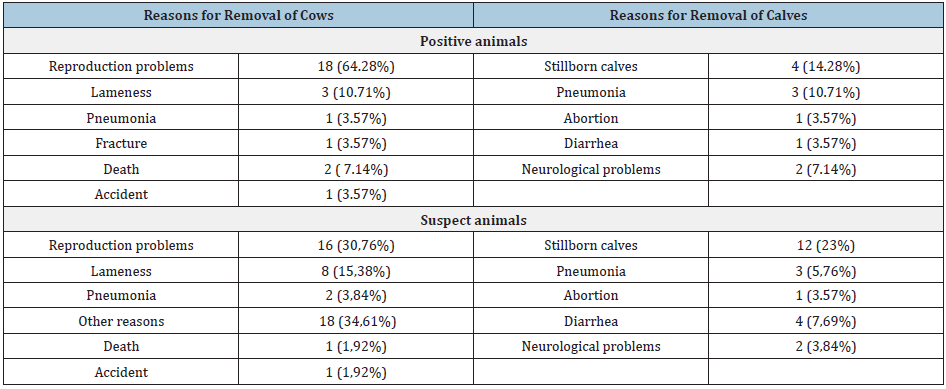
Table 2:Reasons for the removal of positive and suspect animals.
Discussion
The present study recorded a statistically significant higher frequency (P> 0.005) of positive cows manifesting infertility problems (64.28%), compared with suspect cows (30.76%). Moreover, the study showed that infertility problems were the main reason for removal of the animals from the farms. Thus, in farms presenting infertility problems, toxoplasmosis should be included in the differential diagnosis because toxoplasmosis is one of the causes for removal of animals from breeding. In all the farms included in the present study, dogs and cats coexisted with cattle and no preventive antiparasitic treatment was granted to any of the animals. This could be a probable route of infection for cows, since these animal species are known to occasionally harbor and spread Toxoplasma [17]. Risk factors for the contamination of farms are the presence of cats and the accidental consumption of raw infected meat by cows. It is recommended that cats are removed from the farms and that consumption of raw meat is avoided. Preventive examination of all cows should be undertaken every year.
Reproductive abnormalities have a major role in economic losses of the livestock production system, largely as a result of losses of milk production in the dairy sector and of increases in culling rates. It is obvious that there is a need for further evaluation of the economic impacts occurring due to toxoplasmosis in cow farms, and preventive measures for toxoplasmosis should be implemented on cow farms because of both the economic consequences for farmers and the zoonotic importance of the parasitosis. Torgersona and Mastroiacovo [25] estimated that approximately 30% to 50% of people worldwide have an infection with T. gondii [25]. Human infection occurs by two main routes, ingestion of oocysts by fecaloral route and ingestion of undercooked or raw meat containing tissue cysts of the parasite [26,27]. In many countries humans are used to consuming bovine undercooked meat. Therefore, toxoplasmosis must be considered as an issue of public health importance [19,20,27], especially in endemic areas [28].
This study recorded a prevalence of 6.54% positive cows for T. gondii infection. Other studies in several parts of the world record higher prevalence, which notably reaches 9.5% in Asia [29], 27.9% in South Asia [30] and 12% in Africa [31]. In cows the infection is usually asymptomatic or causes mild symptoms [5]. Clinical signs referred are fever, dyspnea, neurological symptoms and abortion [5]. In this study, the related conditions recorded among positive animal were pneumonia in 1 (3.57%) cow and 3 (5.76%) calves, neurological problems in 2 (7.14%) calves and 1 abortion (3.57%). Among suspect cows the results were pneumonia in 2 (3.84%) cows and 3 (5.76%) calves, neurological problems in 2 (3, 84%) calves and 1 aborted fetus (3.57%), respectively. In case of first infection by the parasite in pregnant animals, establishment of a placental and fetal infection is possible, which may result in fetal death and resorption, abortion, or stillbirth [5].
Toxoplasmosis is a common infection in humans, but clinical illness is relatively uncommon [32]. Severe toxoplasmosis, causing damage to the brain, eyes, or other organs, can develop from an acute Toxoplasma infection or one that occurred earlier in life and is now reactivated. At high risk of manifesting serious clinical signs are pregnant women infected for the first time during pregnancy, as the parasite can pose a serious threat to the unborn child [20]. Also, in some individuals who are immunosuppressed, such as tissue transplant patients, AIDS patients, patients with certain types of cancer and those undergoing certain forms of cancer therapy toxoplasmosis could be a cause of serious health problems [33]. People with no apparent immune deficiency may develop an illness characterized by general malaise, fever and mainly lymphadenopathy [1,33,34].
In conclusion, this study recorded the possibility of infertility problems due to toxoplasmosis in dairy cows in Greece and evidenced the need of including this parasitosis in the differential diagnosis because of both the economic losses for farmers and the zoonotic importance for humans. Future research examining the occurrence of Toxoplasma tachyzoites or bradyzoites in cows’ muscle tissue shall be undertaken to expand our knowledge regarding the zoonotic potential of the parasitosis.
Funding
The APC was funded by the University of Thessaly (Special Account for Research Grants).
Ethics Statement
Ethical review and approval were waived for this study since sampling was performed for standard monitoring of the animals’ health and no research on animals, as defined in the EU Ethics for Researchers document (European Commission, 2013, Ethics for Researchers-Facilitating Research Excellence in FP7, Luxembourg: Office for Official Publications of the European Communities, ISBN 978-92-79-28854-8), was conducted.
References
- Dubey JP, Jones JL (2008) Toxoplasma gondii infection in humans and animals in the United States. Int J Parasitol 38(11): 1257-1278.
- Dubey JP (2009) History of the discovery of the life cycle of Toxoplasma gondii. Int J Parasitol 39(8): 877-882.
- Yang N, Ming YM, Hong KL, Miao L, Jian BH (2012) Seroprevalence of Toxoplasma gondii infection in slaughtered chickens, ducks, and geese in Shenyang, northeastern China. Parasites & Vectors 5: 237
- Flegr J, Prandota J, Sovičková M, Israili ZH (2014). Toxoplasmosis - A global threat. Correlation of latent Toxoplasmosis with specific Disease burden in a set of 88 countries. PLoS One 9(3): e90203.
- Toxoplasmosis of Animals and Humans.
- Torrey EF, Yolken RH (2013) Toxoplasma oocysts as a public health problem. Trends Parasitol 29(8): 380-384.
- Sibley LD, Khan A, Ajioka JW, Rosenthal BM (2009) Genetic diversity of Toxoplasma gondii in animals and humans. Philos Trans R Soc Lond B Biol Sci 364(1530): 2749-2761.
- Schlüter D, Däubener W, Schares G, Groß U, Pleyer U, et al. (2014) Animals are key to human Toxoplasmosis. Int J Med Microbiol 304(7): 917-929.
- Yücesan B, Babür C, Koç N, Sezen F, Kılıç S, et al. (2019) Investigation of anti-Toxoplasma gondii antibodies in cats using Sabin-Feldman dye test in Ankara in 2016. Turkiye Parazitol Derg 43: 5-9.
- Veronesi F, Santoro A, Milardi GL, Diaferia M, Morganti, et al. (2017) Detection of Toxoplasma gondii in faeces of privately owned cats using two PCR assays targeting the B1 gene and the 529-bp repetitive element. Parasitol Res 116(3): 1063-1069
- Silaghi C, Knaus M, Rapti D, Kusi I, Shukullari E (2014) Survey of Toxoplasma gondii and Neospora caninum, Haemotropic mycoplasmas and other arthropod-borne pathogens in cats from Albania. Parasites & Vectors 7: 62.
- Spada E, Canzi I, Baggiani L, Perego R, Vitale F, et al. (2016) Prevalence of Leishmania infantum and co-infections in stray cats in northern Italy. Comparative Immunology, Microbiology And Infectious Diseases 45: 53-58.
- Can H, Döşkaya M, Ajzenberg D, Özdemir HG, Caner A, et al. (2014) Genetic characterization of Toxoplasma gondii isolates and Toxoplasmosis seroprevalence in stray cats of izmir, Turkey. Plos One 9(8): e104930.
- Costa GN, Costa AD, Lopes WZ, Bresciani KS, Santos TD, et al. (2011) Toxoplasma gondii: Infection natural congenital in cattle and an experimental inoculation of gestating cows with oocysts. Exp Parasitol 127(1): 277-281.
- Burrells A, Taroda A, Opsteegh M, Schares G, Benavides J, et al. (2018) Detection and dissemination of Toxoplasma gondii in experimentally infected calves, a single test does not tell the whole story. Parasites Vectors 11(1): 45.
- Dubey JP, Thulliez P (1993) Persistence of tissue cysts in edible tissues of cattle fed Toxoplasma gondii Am J Vet Res 54(2): 270-273.
- Stelzer S, Basso W, Silván JB, Mora LO, Maksimov P, et al. (2019) Toxoplasma gondii infection and Toxoplasmosis in farm animals: risk factors and economic impact. Food and Waterborne Parasitology 15: e00037.
- Canada N, Meireles CS, Rocha A, Costa JD, Erickson MW, et al. (2002) Isolation of viable Toxoplasma gondii from naturally infected aborted bovine fetuses. J Parasitol 88(6): 1247-1248.
- Baril L, Ancelle T, Goulet V, Thulliez P, Fleury TV, et al. (1999) Risk factors for Toxoplasma infection in pregnancy: A case-control study in france. Scand J Infect Dis 31(3): 305-309.
- Cook AJ, Gilbert RE, Buffolano W, Zufferey J, Petersen E, et al. (2000) Sources of Toxoplasma infection in pregnant women: European multicentre case-control study. European research network on congenital Toxoplasmosis. BMJ 321(7254): 142-147.
- Rorman E, Zamir CS, Rilkis I, David HBD (2006) Congenital Toxoplasmosis-prenatal aspects of Toxoplasma gondii Reprod Toxicol 21(4): 458-472.
- Saraei M, Shojaee S, Esmaeli A, Hashemi JH, Keshavarz H (2010) Evaluation of confounders in toxoplasmosis indirect fluorescent antibody assay. Iran J Parasitol 5(4): 55-62.
- Sudan V, Tewari AK, Singh H (2019) Detection of antibodies against Toxoplasma gondii in indian cattle by recombinant SAG2 enzyme-linked immunosorbent assay. Acta Parasitol 64(1): 148-151.
- Reichel MP, Drake JM (1996) The diagnosis of Neospora abortions in cattle. New Zealand Veterinary Journal 44: 151-154.
- Torgerson PR, Mastroiacovo P (2013) The global burden of congenital toxoplasmosis: A systematic review. Bulletin of the World Health Organization 91(7): 501-508.
- Guo M, Dubey JP, Hill D, Buchanan RL, Gamble HR et al. (2015) Prevalence and risk factors for Toxoplasma gondii infection in meat animals and meat products destined for human consumption. J Food Prot 78(2): 457-476.
- Cenci-Goga BT, Rossitto PV, Sechi P, McCrindle CME, Cullor JS (2011) Toxoplasma in animals, food, and humans: An old parasite of new concern. Foodborne Pathog Dis 8(7): 751-762.
- Carmo ELD, Morais RDAPB, Lima MDS, Moraes CCGD, Albuquerque GR, et al. (2017) Anti-Toxoplasma gondii antibodies in beef cattle slaughtered in the metropolitan region of belém, brazilian amazon. Rev Bras Parasitol Vet 26(2): 226-230.
- Deng H, Devleesschauwer B, Liu M, Li J, Wu Y, et al. (2018) Seroprevalence of Toxoplasma gondii in pregnant women and livestock in the mainland of china: A systematic review and hierarchical meta-analysis. Sci Rep 8: 6218.
- Khan MU, Rashid I, Akbar H, Islam S, Riaz F, et al. (2017) Seroprevalence of Toxoplasma gondii in south asian countries. Rev Sci Tech 36(3): 981-996.
- Tonouhewa AN, Akpo Y, Sessou P, Adoligbe C, Yessinou, E et al. (2017) Toxoplasma gondii infection in meat animals from africa: Systematic review and meta-analysis of sero-epidemiological studies. Vet World 10(2): 194-208.
- Gangneux RF, Dardé ML (2012) Epidemiology of and diagnostic strategies for toxoplasmosis. Clin Microbiol Rev 25(2): 264-296
- Bhopale GM (2003) Pathogenesis of toxoplasmosis. Comp Immunol Microbiol Infect Dis 26(4): 213-222.
- Durlach RA, Kaufer F, Carral L, Hirt J (2003) Toxoplasmic lymphadenitis-clinical and serologic profile. Clinical Microbiology and Infection 9(7) 625-631.
© 2022 Menelaos Lefkaditis. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






