- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Intestinal Immune Response to Glucan Per Os Supplementation and Low Dose of T-2 Toxin in Lohmann Brown Chickens
Levkut M1, Revajová V1*, Herich R1, Levkutová M2, Karaffová V1, Ševčíková Z1, Paulovičová E3 and Levkut M1
1,2Department of Morphological Disciplines, University of Veterinary Medicine and Pharmacy, Slovakia
3Institute of Chemistry SAS, Slovakia
4Department of Epizootology, Parasitology and Protection of One Health, University of Veterinary Medicine and Pharmacy, Slovakia
*Corresponding author: Viera Revajová, Department of Morphological Disciplines, University of Veterinary Medicine and Pharmacy, Komenského 73, 041 81Košice, Slovakia
Submission: August 24, 2022;Published: September 15, 2022

ISSN: 2576-9162 Volume9 Issue2
Abstract
Immune response of jejunum, ileum and cecum was followed after administration of yeast-derived ß-Dglucan as binder and low dose of T-2 toxin (145.84μg.kg–1) contaminated diet (2 weeks) in chickens. For that purpose, expression of MUC-2, IgA, pIgR genomes, jejunal IgA+ IEL (intraepithelial lymphocytes), and IgA+ LPL (lamina propria lymphocytes) were evaluated. A total of one day old chickens were randomly divided into four groups: control (C), β-D-glucan (G), T-2toxin (T) and β-D-glucan+T-2 toxin (GT). β-Dglucan in jejunum did not influence the activity of MUC-2 and IgA gene expression in combined GT group, but alone T-2 toxin increased their expression. β-D-glucan alone positively influenced intraepithelial IgA+ lymphocytes (p<0.05) in jejunum compared to T group demonstrating suppressed density, and ileal MUC- 2 (p<0.001) compared to C and T group. On the other hand, chickens of GT group showed increased pIgR expression (p<0.001) together with IgA+ IEL and IgA+ LPL density in jejunum. Challenge with T-2 toxin showed beneficial effect of ß-D-glucan on jejunal IgA production. Moreover, immunomodulatory effect of low doses of T-2 toxin on IgA generation was indicated by improving pIgR gene expression in intestine.
Keywords:T-2 toxin; β-D-glucan; Immunity; Small intestine; Chickens
Introduction
Trichothecenes are secondary metabolites of Fusarium moulds, one of the major contaminants of several crops. T-2 toxin is one of the most toxic trichothecene. The immune system is the primary target for trichothecenes and T-2 toxin can both suppress and stimulate immune functions [1] with main affection on the skin and mucous membranes [2]. Its mode of action is time and dose dependent. Immunosuppression is the result of action of high doses that cause damage to the bone marrow, lymph nodes, spleen, thymus and intestinal mucosa [3,4]. Studies on the effect of mycotoxin compounds on intestinal mucosa are limited. The absorption of mycotoxins and their fate within gastrointestinal tract suggests that the epithelium is repeatedly exposed to these toxins, and at higher concentration than other tissues [5]. Mycotoxins alter the different intestinal defence mechanisms including epithelial integrity, cell proliferation, mucus layer and immunoglobulins [6]. The last few years have brought many reports about mycotoxin inactivation agents (e.g. mycotoxin binders) in order to reduce the toxic effects of mycotoxins in animals. Mycotoxin binders are used in the mineral form (inorganic) as for example active charcoal, sodium bentonite, dietary clay and zeolites [7]. The application of microorganisms capable of bio transforming certain mycotoxins into less toxic metabolites have been proposed [8,9]. The microorganisms act in the intestinal tract of animals prior to the absorption of the mycotoxins. Many species of bacteria and fungi have been shown to enzymatically degrade mycotoxins [10]. Yeast or yeast cell walls show a potential as organic mycotoxin binders. The cell walls harbouring polysaccharides (glucan, mannan), proteins, and lipids exhibit numerous different and easily accessible adsorption centres including adsorption mechanisms [11].
To maintain intestinal homeostasis, the secretory immune system of mucosa, secretory IgA with flowing mucus, form the first immunological barrier of defence to limit epithelial contact with and penetration by intestinal microbiota and other potentially dangerous contaminants [6]. The major component of intestinal mucus is secretory mucin 2 (MUC-2) produced by goblet cells that is in direct contact with gut bacteria [12-14]. Polymeric IgA (pIgA) secreted by plasma cells accumulates in the lamina propria. To exert its protective effect, pIgA is transported to the intestinal lumen. This process is mediated by the polymeric immunoglobulin receptor (pIgR), which is expressed on the basolateral surface of epithelial cells [15]. There are few scientific papers that investigate the influence of T-2 toxin as the most potent and cytotoxic trichothecene and organic binder (glucan) on the chicken intestinal immunological barrier in the low dose. From that reason we decided to study the intestinal mucosal immunity in the chicken fed during two weeks with low dose of T-2 toxin contaminated diet and perorally administered β-D-glucan as a potential binder of mycotoxin. For that purpose, MUC-2, IgA with pIgR gene expression, and quantified IgA+ intraepithelial (IEL) and Lamina Propria Lymphocytes (LPL) were evaluated.
Material and Methods
Chickens, housing and nutrition
Table 1: Chemical composition of the experimental diet.
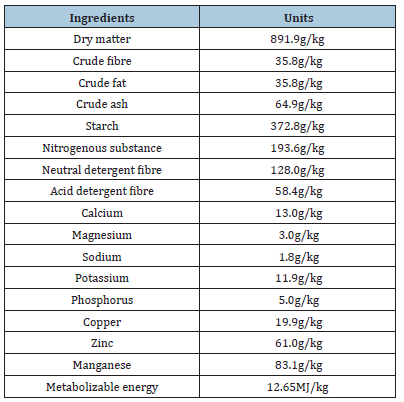
Forty-one-day-old chickens of Lohmann Brown hybrid were used. They were allocated to four groups: control (C), β-D-glucan (G), β-D-glucan+T-2 toxin (GT), T-2 toxin (T), and were placed in wire cages (n=10) with solid floors. All chickens were fed a free of antimycotics diet prepared at Department of Pathological Anatomy (UVMP, Košice, SK) corresponding to commercial HYD-02/a diet for chickens. Composition of the diet included next ingredients: maize 45%, wheat 11%, soya 35%, rape seal oil 4%, feed calcite 2%, premix 2%, lysine 1%. Content of nutrients given in g/kg showed Table 1. The chicks were kept in the menagerie of the Department of Pathological Anatomy, University of Veterinary Medicine and Pharmacy, Košice, Slovakia (SK P 52004), in accordance with the rules and approval of the Ethics Committee, and the experiment was authorized by the State Veterinary and Food Administration of the Slovak Republic (Č.k.Ro-270710-221).
Preparing of β-D-glucan and T-2 toxin, experimental desig
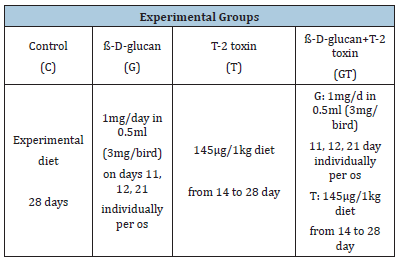
Beta-D-glucan in lyophilized powder form was obtained from Candida albicans (Chemical Institute of SAS, Bratislava, Slovak Republic). One hundred mg was dissolved in Aqua pro injectione with final concentration 3mg.ml–1. Beta-D-glucan was individually per os administrated to chickens in dose 0.5ml (1.0mg/birds/day). T-2 toxin OEKANAL® in powder form (mol. w. 466.52g.mol–1, Sigma- Aldrich, Germany) was dissolved in 50ml of 96% ethanol; 2ml was added into 1kg of diet and very well mixed with hand-mixer. Concentration 145.84μg.kg-1 of food was proved by HPLC method in State Veterinary and Food Institute, Bratislava, Slovak Republic (SNAS Reg. No. 050/S-127) together with other mycotoxins (Table 2). The experiment lasted 28 days and was carried out under the proposed scheme (Table 3). Group C served as a negative control, chickens in groups G and combined GT were individually per os administered with β-D-glucan on days 11, 12 and 21. The daily prepared contaminated diet with T-2 toxin (145.84μg.kg–1) was given ad libitum to T and GT groups between 14 and 28 days. Intestinal samples were collected from chickens slaughtered on day 28, after consuming contaminated diet with toxin during 2 weeks, by euthanasia and capitation.
Table 2:Mycotoxin content of the experimental diet determined by HPLC method.
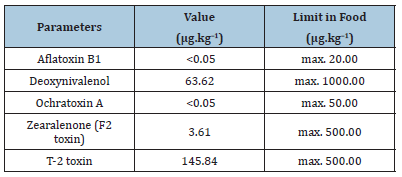
Table 3 :The scheme of experiment.
Flow cytometry - immunophenotyping of IEL and LPL
Lymphocytes from jejunal mucosa (IEL, LPL) isolated and purified by the modified method of Solano-Aguilar et al. [16] was described in more detailed previously by Bucková & Revajová [17]. Briefly, the jejunum placed in ice cold buffered Hank´s solution (HBSS, pH 7.2-7.3) was cut lengthwise into 0.5cm pieces and transfer into 50ml plastic tubes with 5mM dithiothreitol (HBSSDDT) 37 °C for removing of mucin during 15min in thermostat. After 3 times rinsing in cold HBSS incubation of jejunum for 1 hour in 0.1mM EDTA-HBSS at 37 °C released Intraepithelial Lymphocytes (IEL). Supernatant was filtered through a nylon sieve and place in refrigerator. The pieces were rinsed with RPMI-1640 (Sigma, Germany) and incubated for 1 hour with collagenase-RPMI (15mg.30ml-1; Collagenase Type I, HP Biomedicals LLC, France) to obtain Lamina Propria Lymphocytes (LPL). Both supernatants with isolated IEL and LPL were centrifuged 10min at 600g, sediment was 3 times rinsed with PBS. The number of lymphocytes was determined in a Bürker chamber using a Türk solution (diluted 1:20) and the concentration was adjusted to 1x106.50μl-1. Direct immunofluorescence was used for immunophenotyping of IgA+ lymphocytes. The concentration of lymphocytes 1×106 in 50μl of PBS and 2μl of IgA PE labelled mouse anti-chicken monoclonal antibody (Southern Biotech, USA) were used. Mouse IgG 2b R-PE isotype control was used. The cells were incubated at room temperature in dark for 15min, rinsed with 1ml PBS (1400rpm, 5min) and next diluted with 200μl PBS+1% paraformaldehyde. The lymphocytes (10,000) were measured and analysed by flow cytometer FACScan with Cell Quest software (both BD, Germany). The positive IgA subpopulation was expressed in relative percentages [18].
Tissue homogenization, isolation of total RNA and RTPCR
The samples from intestine (jejunum, ileum, cecum) were taken up during the necropsy and placed into RNA later (Qiagen, UK). Intestinal fragments were transferred into 1ml of TRI Reagent® RT for In vitro research use (Molecular Research Centre, USA), homogenized with zirconia silica beads (BioSpec Products, USA) and mixing in vortex. Samples were removed and in order to separate the phases, 50μl of 4-bromanisole were added (Molecular Research Centre, USA). Samples of tissue were centrifuged at 12 000 rpm for 15min. at 4 ºC. After centrifugation upper aqueous phase was collected and replaced into new tubes for total RNA isolation using the RNA easy mini kit (Qiagen, UK). RNA quality and quantity were measured with Nano Drop 200c UV-Vis spectrophotometer (Thermo Scientific, USA). Isolated RNA was immediately reverse transcribed by using iScript cDNA Synthesis Kit (Bio-Rad, USA). The resulting cDNA was diluted 10x in Ultra Pure™ DNase/RNase-free distilled water (Invitrogen, USA) and used as a template in realtime PCR. Quantity and purity of cDNA were assessed using Nano Drop 200c UV-Vis spectrophotometer. The primer sequences used for qPCR are listed in Table 4. RT-PCR was performed using Thermo Scientific Maxima First Strand cDNA Synthesis kit for RT-qPCR # K1642 (Thermo Fisher Scientific Inc., USA) using thermocycler CFX 96 RT (Bio-Rad, USA). Briefly, 12.5μl of Supermix, 0.3μl of forward primer (100ngμl-1), 0.3μl of reverse primer (100ng.μl- 1), 2.0μl of cDNA and DEPC water were added to total volume of 25μl. Amplification conditions in case of IgA were done: initial denaturation at 95 ºC for 15min, followed by 40 cycles denaturation at 95 ºC for 20s, annealing of IgA at 60 ºC for 30s, and elongation at 72 ºC for 30s. In case of MUC2 the amplification conditions were slightly different: denaturation at 95 ºC 30s, annealing at 54 ºC 1min and extension at 72 ºC for 1min. Samples were amplified in duplicates. The recorded data were normalized to reference gene GAPDH (glyceraldehyd-3-phosphate dehydrogenase). The relative expression ratio of genes was calculated and based on the Ct comparative threshold cycle method. The values of genes of interest were normalized to an average Ct value of reference genes (ΔCq) and relative expression of each representative was calculated as 2 delta Cq.
Table 4 :List of primers used in qRT-PCR for MUC-2 and IgA, PIgR mRNA detection in chickens.

Statistical analysis
Data obtained from six animals (n=6) of each group were tested by one-way ANOVA with Tukey post-test by Minitab 16 software (SC&C Partner, Brno, Czech Republic) to determine mean values, standard deviations and significant differences. A p value <0.05 was considered to be significant.
Results
MUC-2 gene expression
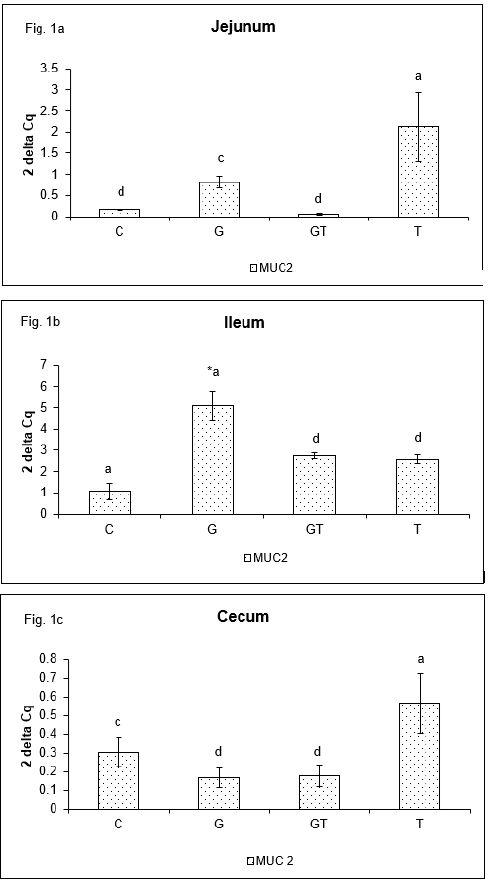
After two weeks of the low dose T-2 toxin administration, the relative mRNA levels of MUC-2 were not consistent in studied parts of the small intestine in chickens. In the jejunum (Figure 1a) mRNA showed higher expression of MUC-2 gene in T group comparing to GT and control (p<0.001) groups, as a G (p<0.01) group. In the ileum (Figure 1b) expression of MUC-2 gene was higher in G, GT and T groups (p<0.001) in comparison with control group. Moreover, expression of MUC-2 gene was higher in G group than in GT and T (p<0.001) groups. The cecum (Figure 1c) demonstrated increased expression of MUC-2 gene in T group than in GT and G groups (p<0.001), and in controls (p<0.01).
Figure 1: Relative expression of MUC-2 gene in jejunum (a), ileum (b) and cecum (c) of chickens after administration of ß-D-glucan and low dose of T-2 toxin. Means with different superscripts are significantly different, acp<0.01, adp<0.001, *dp<0.001.
IgA gene expression
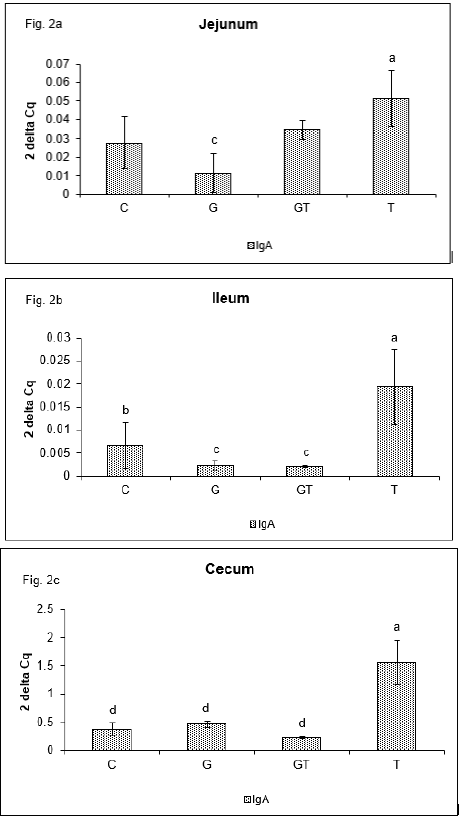
Similarly, to MUC-2, the different levels of relative IgA gene expression were detected in the evaluated sections of intestine [19]. In the jejunum the relative mRNA level of IgA (Figure 2a) was higher (p<0.01) in T group than in G group and showed tendency to increase in GT group in comparison with C and G groups. In the ileum (Figure 2b) expression of IgA gene was higher in T group than in G and GT groups (p<0.01), and controls (p<0.05). The similar mRNA levels of IgA gene expression were seen in the cecum (Figure 2c) where T group showed higher (p<0.001) expression than other groups.
Figure 2: Relative expression of IgA gene in jejunum (a), ileum (b) and cecum (c) of chickens after administration of ß-D-glucan and low dose of T-2 toxin. Means with different superscripts are significantly different, abp<0.05, acp<0.01, adp<0.001.
PIgR gene expression
PIgR gene expression in the jejunum (Figure 3a) was higher in GT group (p<0.001) than in other groups. By contrast, in the ileum (Figure 3b) the lower expression was found in G and GT group than in control (p<0.001) and T (p<0.01) groups. In the cecum (Figure 3c) pIgR expression was higher in T group (p<0.001) than in other groups.
Figure 3: Relative expression of pIgR gene in jejunum (a), ileum (b) and cecum (c) of chickens after administration of ß-D-glucan and low dose of T-2 toxin. Means with different superscript are significantly different, acp<0.01, adp<0.001.

Relative percentage of jejunal IgA+ IEL and LPL
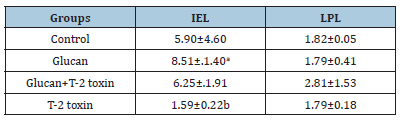
The results summarized in Table 5 showed higher number of jejunal intraepithelial IgA+ lymphocytes in G group (p<0.05) than in T group. Also, the number of GT group was insignificantly higher than T-2 toxin and control groups. On the other hand, jejunal lamina propria IgA+ lymphocytes showed the maximal density in GT group of chickens.
Table 5 :Relative percentage of jejunal IgA positive intraepithelial (IEL) and Lamina Propria Lymphocytes (LPL) at 28 days of the experiment (n=6; mean±SD). Different superscripts in columns are significantly different - abp<0.05.
Discussion
Viability and proliferation of animal and human intestinal epithelial cells can be negatively affected by Fusarium mycotoxins [6,20]. Following oral intake of low to moderate amounts of Fusarium mycotoxins, the gastrointestinal epithelial cell layer is exposed first [21]. In current trial the administration of low dose T-2 toxin contaminated diet to chickens increased expression of jejunal MUC-2 and IgA gene expression. It was interesting to find improved expression of ileal MUC-2 gene in G group. On the other hand, administration of β-D-glucan did not increase the jejunal IgA gene expression in combined GT group up to T-2 toxin level. Previously, Rezar et al. [22] reported increase of serum IgA antibodies after low doses T-2 toxin administration in diet of chickens. In our experiment the highest level of IgA gene expression in T-2 toxin groups was measured in all examined segments of intestine. However, our results did not show the changes in ileal and cecal IgA gene expression in combined GT group. Explanation of this difference can be that the majority of ingested mycotoxins are absorbed in the proximal part of the gastrointestinal tract [23] or binding efficiency of T-2 toxin to purified β-D-glucan [24]. Immunoglobulin A provides mucosal immune protection as a result of its ability to interact with the polymeric Ig receptor, an antibody transporter, expressed on the basolateral surface of epithelial cells [25]. The pIgR is constitutively expressed at quite high level particularly in the small and large intestine [26]. This prompted us to examine the expression of that genome and find possible changes in its regulation by low dose of T-2 toxin or administered binder of glucan which could influence the transmission of IgA on the intestinal epithelial surface.
Current trial demonstrated increase of jejunal pIgR gene expression in combined GT group. Distal intestinal part showed interestingly increased expression of that gene in T-2 group. It is supposed that in proximal intestinal part played an important role symbiotic effect of β-D-glucan as enhancer of natural and acquired immunity, and T-2 toxin with its stimulating effect in low doses. In previous work Revajová et al. [27] found increased number of actively dividing intestinal cells in chickens during administration of low dose of T-2 toxin, what suggests its stimulating effect on proliferation of epithelial cells. Increased jejunal pIgR gene expression in GT group correlated with increased number of jejunal IgA+ intraepithelial and lamima propria lymphocytes. On the other hand, higher cecal pIgR gene expression in T-2 group correlated with cecal expression of IgA genome. Immunostimulation of both genomes suggests their overstimulation for intestinal secretory IgA (sIgA). Comparison of pIgR, MUC-2 and IgA gene expression in examine intestine segments showed their highest activity in cecum of T group. This phenomenon suggests being caused by retained contents in cecum for longer period than would be possible in the main intestine through which digesta move relatively rapidly [28]. Higher, although no significant number of intraepithelial IgA+ lymphocytes in β-D-glucan administered group of chickens was observed in comparison with low dose of T-2 toxin group. It is known that β-glucans increase host immune defence by activating complement system, enhancing macrophages and natural killer cell function [29]. Zhang et al. [30] found increased level of plasma IgA after feeding of β-glucan. Similarly, Buts et al. [31] observed in rats’ intestine the stimulation of sIgA production after treatment with Saccharomyces boulardii. In current trial increased number of IgA+ lymphocytes suggest higher activation of IgA production what could be consistent with increased level of sIgA. On the other hand, feeding of chickens with T-2 toxin contaminated diet in our experiment not suppressed the beneficial effect of β-D-glucan on jejunal IgA+ lymphocytes.
Most of the experiments evaluated the effect of binders on the bases of morphological, biochemical, haematological or plasma proteins parameters in blood [32,33]. Girish & Devegowda [34] reported that organic binder (glucomannan) improved antibody titres against Newcastle disease virus and infectious bursal disease virus. The current results indicate immunological changes after administration of β-D-glucan already in first barrier of intestinal immunity. Previous work of Bailey et al. [35] and Girish & Devegowda [34] did not observe any significant protection by the inorganic sorbent against the effects of T-2 toxin in chickens. On the other hand, Garcia et al. [33] suggest that T-2 toxin toxicity could be partially counteracted by inorganic binders. It appears that organic binders have better ability for immunomodulatory effect in chicken fed T-2 toxin contaminated diet than inorganic binders [36]. In conclusion, these data suggest that dietary administration of yeast-derived β-D-glucan did not influence the activity of MUC-2 and IgA gene expression in Lohmann Brown hybrid fed low dose T-2 toxin contaminated diet (GT group) in jejunum, ileum and cecum. On the other hand, alone administration of low dose T-2 toxin contaminated diet increased expression of followed genes. Immunostimulatory effect of T-2 toxin confirmed correlation of pIgR gene expression with expression of IgA genome in ileum and cecum. Beta-D-glucan positively influenced the number of jejunal intraepithelial IgA+ lymphocytes and ileal MUC-2 in G group, as well as IgA+ IEL and LPL in GT groups. Challenge with T-2 toxin showed beneficial effect of ß-D-glucan on jejunal IgA production. Moreover, immunomodulatory effect of low dose of T-2 toxin on IgA generation was indicated by improved pIgR gene expression in intestine.
Acknowledgement
This research was funded by the Grant Agency for Science of the Slovak Republic VEGA 1/0885/11, 1/0355/19, 1/0107/21, Slovak Research and Developmental Agency APVV-15-0165.
References
- Sklan D, Shelly M, Makovsky B, Geyra A, Klipper E, et al. (2003) The effect of chronic feeding of diacetoxyscirpenol and T-2 toxin on performance, health, small intestinal physiology and antibody production in turkey poults. Br Poult Sci 44(1): 46-52.
- Yang Y, Li G, Wu D, Liu J, Li X, et al. (2020) Recent advances on toxicity and determination methods of mycotoxins in foodstuffs. Trends Food Sci Technol 96: 233-252.
- Sharma RP (1993) Immunotoxicity of mycotoxins. J Dairy Sci 76(3): 892-897.
- Holladay SD, Smith BJ, Luster MI (1995) B-lymphocyte precursor cells represent sensitive targets of T2 mycotoxin exposure. Toxicol Appl Pharmacol 131(2): 309-315.
- Grenier B, Applegate TJ (2013) Modulation of intestinal functions following mycotoxin ingestion: Meta-analysis of published experiments in animals. Toxins 5(2): 396-430.
- Gao Y, Meng, L, Liu, H, Wang, J, Jheng, N (2020) The compromised intestinal berrier induced by mycotoxins. Toxins 12(10): 619.
- Tomaševič-Čanovič M, Dakovič A, Rottinghaus G, Matijaševič D, Duričič K (2003) Surfactant modified zeolites-new efficient adsorbents for mycotoxins. Microporous and Mesoporous Materials 61(1-3): 173-180.
- Jouany JP, Yiannikouris A, Bertin G (2005) The chemical bonds between mycotoxins and cell wall components of Saccharomyces cerevisiae have been identified. Archiva Zootechnica 8: 26-50.
- Shetty HP, Jespersen L (2006) Saccharomyces cerevisiae and lactic acid bacteria as potential mycotoxin decontaminating agents. Trends Food Sci Technol 17(2): 48-55.
- Avantaggiato G, Havenaar R, Visconti A (2004) Evaluation of the intestinal absorption of deoxynivalenol and nivalenol by an in vitro gastrointestinal model, and the binding efficacy of activated carbon and other absorbent materials. Food Chem Toxicol 42(5): 817-824.
- Huwig A, Freimund S, Käppeli O, Dutler H (2001) Mycotoxin detoxication of animal feed by different adsorbents. Toxicol Lett 122(2): 179-188.
- Smirnov A, Perez R, Amit-Romach E, Sklan D, Uni Z (2005) Mucin dynamics and microbial populations in chicken small intestine are changed by dietary probiotic and antibiotic growth promoter supplementation. J Nutr 135(2): 187-192.
- Smirnov AE, Tako PR, Ferket PR, Uni Z (2006) Mucin gene expression and mucin content in the chicken intestinal goblet cells are affected by in ovo feeding of carbohydrates. Poult Sci 85(4): 669-673.
- McGuckin MA, Lindén SK, Sutton P, Florin TH (2011) Mucin dynamics and enteric pathogens. Nat Rev Microbiol 9(4): 265-278.
- Zhang Q, Eicher, SD, Applegate TJ (2015) Development of intestinal mucin 2, IgA, and polymeric Ig receptor expressions in broiler chickens and Pekin ducks. Poult Sci 94(2): 172-180.
- Solano-Aguilar GI, Vengroski KG, Beshah, E, Lunney JK (2000) Isolation and purification of lymphocyte subsets from gut-associated lymphoid tissue in neonatal swine. J Immunol Meth 241(1-2): 185-199.
- Bucková B, Revajová V (2014) Immunophenotyping of Intraepithelial (IEL) and Lamina Propria Lymphocytes (LPL) in the chicken intestine by flow cytometry. Folia Veterinaria 58(2): 75-77.
- Lammers A, Wieland WH, Kruijt L, Jansma A, Straetemans, T, et al. (2010) Successive immunoglobulin and cytokine expression in the small intestine of juvenile chicken. Dev Comp Immunol 34(12): 1254-1262.
- De Boever S, Vangestel C, De Backer P, Croubels S, Sys SU (2008) Identification and validation of housekeeping genes as internal control for gene expression in an intravenous LPS inflammation model in chickens. Vet Immunol Immunopathol 122(3-4): 312-317.
- Yunus AW, Blajet-Kosicka, A, Kosicki R, Khan MZ, Rehman H, et al. (2012) Deoxynivalenol as a contaminant of broiler feed: Intestinal development, absorptive functionality and metabolism of the mycotoxin. Poult Sci 91(4): 852-861.
- Bouhet S, Oswald IP (2005) The effect of mycotoxins, fungal food contaminants, on the intestinal epithelial cell-derived innate immune response. Vet Immunol Immunopathol 108(1-2): 199-209.
- Rezar V, Frankič M, Narat M, Levart A, Salobir J (2007) Dose-dependent effect of T-2 toxin on performance, lipid peroxidation, and genotoxicity in broiler chickens. Poult Sci 86(6): 1155-1160.
- Cavret S, Lecoeur S (2006) Fusariotoxin transfer in animal. Food Chem Toxicol 44(3): 444-453.
- Bzducha-Wróbel A, Bryla M, Gientka I, Blažejak S, Janowicz M (2019) Candida utilis ATCC 9950 cell walls and β(1,3)/(1,6)-glucan preparations produced using agro-waste as a mycotoxins trap. Toxins 11(4): 192.
- Cerutti A, Rescigno M (2008) The biology of intestinal immunoglobulin a responses. Immunity 28(6): 740-750.
- Johansen FE, Brandtzaeg P (2004) Transcriptional regulation of the mucosal IgA system. Trends Immunol 25(3): 150-157.
- Revajová V, Herich R, Levkut M Jr, Žitňan R, Albrecht E, et al. (2018) Imunolocalization of Na+/K+-ATPase and proliferative activity of enterocytes after administration of glucan in chickens fed T-2 toxin. Acta Vet Brno 87(4): 371-377.
- Clench MH, Mathias JR (1995) The avian cecum: a review. Wilson Bull 107(1): 93-121.
- Akramiené D, Kondrotas A, Didžiapetriené J, Kevelaitis E (2007) Effects of β-glucans on the immune system. Medicina 43(8): 597-606.
- Zhang B, Guo Y, Wang Z (2008) The modulating effect of β-1, 3/1, 6-glucan supplementation in the diet on performance and immunological responses of broiler chickens. Asian-Austr J Anim Sci 21(2): 237-244.
- Buts JP, Bemascono P, Vaeman JP, Dive C (1990) Stimulation of secretory IgA and secretory component of immnoglobulins in small intestine of rats treated with Saccharomyces boulardii. Dig Dis Sci 35(2): 251-256.
- Aravind KL, Patil VS, Devegowda G, Umakantha B, Ganpule SP (2003) Efficacy of esterified glucomannan to counteract mycotoxicosis in naturally contaminated feed on performance and serum biochemical and hematological parameters in broilers. Poult Sci 82(4): 571-576.
- Garcia AR, Avila E, Rosiles R, Petrone VM (2003) Evaluation of two mycotoxin binders to reduce toxicity of broiler diets containing ochratoxin A and T-2 toxin contaminated grain. Avian Dis 47(3): 691-699.
- Girish CK, Devegowda G (2006) Efficacy glucomannan-containing yeast product (Mycosorb) and hydrated sodium calcium aluminosilicate in preventing the individual and combined toxicity of aflatoxin and T-2 toxin in commercial broilers. Asian-Aust J Anim Sci 19(6): 877-883.
- Bailey RH, Kubena LF, Harvey RB, Buckley SA, Rottinghaus GE (1998) Efficacy of various norganic sorbents to reduce the toxicity of aflatoxin and T-2 toxin in broiler chickens. Poult Sci 77(11): 1623-1630.
- Kurochkina AS, Krasnoshtanova AA (2020) Study of the sorption capacity of soluble forms of yeast beta-glucan. Butlerov Communications 63(9): 43-50.
© 2022 Revajová V. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






