- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Effects of Ginger Protease on Quality of Mozzarella Cheese
Adhikari BR1,2*, Bhattarai RR3, Katuwal N4 and Das SKL1
1Central Department of Food Technology, Central Campus of Technology, Tribhuvan University, Nepal
2Food Management and Trading Company Limited, Provincial Office, Nepal
3School of Molecular and Life Sciences, Faculty of Science and Engineering, Curtin University, Bentley, Australia
4Food Technology and Quality Control Office, Nepal
*Corresponding author: Basanta Raj Adhikari, Central Department of Food Technology, Central Campus of Technology, Tribhuvan University, Hattisar, Food Management and Trading Company Limited, Provincial Office, Biratnagar, Morang, Nepal
Submission: December 07, 2021;Published: December 20, 2021

ISSN: 2576-9162 Volume8 Issue5
Abstract
The study investigated the potential use of ginger protease as a coagulant in the preparation of mozzarella cheese. Control cheeses were prepared using calf rennet for comparison. Numerical optimization study revealed maximum milk clotting activity at pH5, temperature 35°C and enzyme concentration 15μL/mL of milk using ginger protease. Fat, ash, acidity, pH, calcium content and yield of the cheese were similar to the control. Ginger protease significantly enhanced the flavor of the cheese. While meltability and baking properties were comparable to the control, stretchability was relatively lower.
Keywords: Milk clotting; Plant rennet; Casein; Mozzarella; Coagulation; Purification
Abbreviations: BSE: Bovine Spongiform Encephalopathy; PA: Proteolytic Activity; MCA: Milk-Clotting Activity; RSM: Response Surface Methodology; ANOVA: Analysis of Variance; LSD: Least Square Difference; DAR: Direct Acidification Rennet; SCR: Starter Culture Rennet; SCGP: Starter Culture Ginger Protease
Introduction
Cheese is a valuable and nutritious dairy product containing a complex matrix of casein,
fat, lactose, minerals and water [1]. The coagulation of milk protein (casein) is caused
by the action of proteolytic enzymes from animal or plant origin [2]. There are 500-800
varieties of cheese available in the international market [3]. In Nepal, the important varieties
produced are yak cheese, Kanchan cheese, mozzarella like cheese and processed cheese [4].
Mozzarella cheese is a soft, unripened cheese variety of the pasta-filata family, which had its
origin in the Battipaglia region of Italy [5]. The demand for mozzarella cheese is increasing
due to the expansion of pizza parlors and fast food chains and it is more suitable for pizza
topping [6]. The most popular milk-coagulating enzyme is found in the stomach of infant
animals and is known as rennet [7]. Rennet contains chymosin that causes coagulation of
milk by cleavage of the bond between Phe105-Met106 linkage of k-casein [8]. Extraction and
subsequent purification of calf rennet from the tissues of animal stomach require various
steps, which makes this enzyme very expensive. Moreover, the reduced supply of calf rennet
and calf diseases, like Bovine Spongiform Encephalopathy (BSE), have led to an increase in
the demand for alternatives sources of milk coagulants [9,10]. The consumer constraints
on the use of animal rennet for dietary (e.g. vegetarianism), religious as well as concerns
over genetically engineered foods (e.g., Germany, Netherlands and France forbid the use of
recombinant calf rennet). This led to a growing interest in vegetable-based coagulants [11].
Different plant proteases like papain, bromelin, ficin, oryzasin, cucumisin, sodom apple and Jacaratia corumbensis have been identified in different parts of
the world as an alternative to animal rennet [12]. Enzymes from
plant extracts hydrolyse the κ- and β casein, leading to the curd
formation [10]. The use of plant proteinases as milk coagulants is
interesting because they are the natural enzymes and can produce
cheeses suitable for lacto-vegetarian consumers and ecological
markets [13]. Recent publications on new milk clotting proteolytic
enzymes from the vegetable origin [14-17] revealed that vegetable
coagulants are gaining growing interest in the dairy industry. These
plant coagulants have been used for the preparation of different
varieties of cheeses viz. French cheeses as Camembert and Gruy_
ere, Gu_õa cheese, Ovine cheeses, Warankashi and Tofu [18-22].
One of the potential plants that possesses proteolytic enzymes
is ginger. Ginger protease is a cysteine protease characterized by a
cysteine residue at the active center of the enzyme [23]. Majority
of previous studies focused on the Proteolytic Activity (PA),
purification, structural analysis, and meat tenderization properties
of this enzyme [24,25]. The traditional use of ginger protease in
producing milk curd has also been reported [26]. Ginger protease
is, therefore, a rennet like enzyme that exhibits a strong coagulating
activity and hydrolyzes α, β, and κ-casein, and have potential in
preparing cheese and oriental dairy foods [27]. Unlike rennet,
plant-derived protease possess broad substrate specificity and
most of them are classified as cysteine proteases [28]. Chymosin
from animal origin, on the other hand, hydrolyze the κ-casein at the
Phe105-Met106 bond during the primary phase of milk curdling
and destabilize the casein micelles, resulting in milk gelation
[29,30]. This study aimed to investigate the feasibility of ginger
protease on milk clotting potentiality during mozzarella cheese
making. The physicochemical, functional and sensory parameters
were analysed to determine technological suitability of ginger
protease for mozzarella cheese production.
Materials and Methods
The work was conducted in the Central Department of Food Technology, Central Campus of Technology, Sunsari, Nepal. Standardised milk was obtained from a local dairy, Nepal. The calf rennet and mixed culture consisting of streptococcus salavarious subsp. thermophillus and Lactobacillus delbrueckii subsp. bulgaricus were bought from Trisuli Traders, Kathmandu, Nepal. The ginger rhizome (Zingiber officinale var. Nashe) was collected from the local market of Dharan, Nepal.
Extraction of crude ginger protease extracts
The ginger protease was extracted from fresh ginger rhizomes using the method of Haliu [31] with some modifications. Briefly, the ginger rhizome was washed with de-ionized water, peeled and chopped to 2-3mm size. It was then homogenized using a blender (Model 38BL40, Blender 8010E, Christiano Scientific Equipment, USA) with 5 parts of cold acetone (w/v) (-23°C) and kept at 4°C for 20min. The homogenate was filtered through a cotton cloth and the residue was further washed with cold acetone. The filter cake was dried in a forced air oven at 40°C until no acetone odor was detectable. The air-dried material was powdered using a food grade-grinding mill (Model M20, KIKA®WERKW, Germany) and passed 100% through 500um sieve. The powder was homogenized in 200mL of 100mM citrate phosphate buffer (pH 7.0) for 2.4 min and the extract was filtered using a muslin cloth. The filtrate was centrifuged at 12,0000-×g for 20min and the supernatant was recovered as a crude extract.
Milk clotting activity
Milk-Clotting Activity (MCA) was measured using the standard assay procedure as described by Berridge [32] with slight modifications. Here, the pH of 4-6 was adjusted in 1.5L of standardized milk and 10-20μL of enzyme solution per mL of milk was added at 40°C. The time required to form curd fragments was measured. MCA, expressed in Otani units, was calculated as follows:
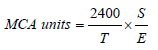
Where:
T=Time (in sec) necessary for the curdling of milk
S=Volume (in mL) of milk
E=Volume (in mL) of enzyme.
Experimental design
The effect of three independent variables, namely enzyme concentration (10-20μL/mL of milk, X1), and pH (4-6, X2) and temperature (30-40°C, X3) of milk on MCA of extracted protease from ginger rhizomes was investigated using Response Surface Methodology (RSM). The independent variables and their levels were selected based on literature and preliminary experiments. A three-factor, five-level central composite rotatable design was employed. MCA was the response variable in this design. The experimental design, data analysis and quadratic modeling were performed using the “Design Expert” software (Version 6.0.1, Stat- Ease Inc., USA). The responses MCA for different experimental combinations were related to the coded variables (xi, i=1, 2and 3) by a second-degree polynomial equation:
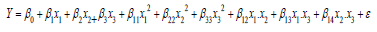
The coefficients of the polynomial were represented by β0 (constant), β1, β2, β3 (linear effects); β12, β13, β14 (interaction effects); β11, β22, β33 (quadratic effects); and ε (random error). Data were modeled by multiple regression analysis. A complete second-order quadratic model employed to fit the data and adequacy of the model was tested considering R2 (the coefficient of multiple determination, a measure of the amount of variation around the mean explained by the model), Adjusted R2 (a measure of the amount of variation around the mean explained by the model, adjusted for the number of terms in the model), predicted R2 (a measure of how good the model predicts a response value) and Fischer’s F-test. The significances of all terms in the polynomial were judged statistically by computing the F-value at probability (p) of 0.05.
Preparation of mozzarella cheese
Four different experiments were undertaken to prepare mozzarella cheese using calf rennet and ginger protease employing two methods-starter culture and direct acidification. The experiments were performed in triplicates, and the mean was taken for analysis. Control cheese was prepared using calf rennet by starter culture [6] and direct acidification Panthi [33] methods. For ginger protease, the cheese-making method was modified as follows: One and a half liters of standardized milk was pasteurized at 72oC for 15s and cooled to 35oC. Milk pH was brought to 5 with 2.4 per cent (w/v) solution of citric acid or by addition of starter culture with agitation for two different methods. The crude enzyme was added at the rate of 15μL/mL of milk and mixed thoroughly. The coagulum was obtained at 45min. After cutting, the curd was cooked in whey to the temperature of 56oC and then separated from the whey. Sufficient quantity of hot water (85oC) was added to cover the curd for 5min. Hot water was drained and the curd was then plasticized manually with a wooden worker to gain elasticity. The curd was moulded in round blocks, salted at the rate of 1.75% and dipped in pasteurized chilled water (4oC) for 1 hr. Cheese blocks were then placed on stainless steel wire mesh at 10oC for 2hr to remove excess water. Hence prepared mozzarella cheese was packed in clean, sanitized polyethylene bags and stored at 5oC before analysis.
Physicochemical analyses
Samples of mozzarella cheese were analysed for pH and titratable acidity, as well as moisture, protein, calcium and ash contents according to the Official methods of AOAC [34]. Fat was determined using Gerber method [35]. The theoretical yield (Y) was calculated using Van Slyke yield equation [36]:
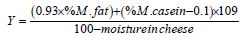
Where
%M. fat and casein refers to %fat and casein in milk
The 0.93x milk fat assumes that 93% of milk fat is retained in
the cheese. The value for casein -0.1 approximates to a theoretical
loss of 4% casein and casein retention of approximately 96%. The
109 is a ‘constant’ to allow for milk salts retention of whey protein
and lactose. Actual yield was calculated by weighing the curd after
pressing as described by Razzaq [3].
Determination of meltability of cheese
A suitable modification to ‘Schrieber test’ Muthukumarappan [37] was made for testing of the mozzarella cheese meltability. For simplicity and wider applicability, the bored sample having the fixed base area (1.75cm2) and height (0.5mm) was heated on the aluminum dish in the boiling water for 5min. The whole assembly was covered with the plate during heating allowing the steam to escape. The dish was then taken out at the room temperature and cooled. The increase in melted cheese area was measured on the graph paper. The ratio of the melted cheese area to the original area was regarded as the indicator of cheese meltability.
Determination of stretchability of cheese
Stretchability test was based on the principle of ‘stretch test’ as described by Bhattarai and Acharya 2010 [6]. The stretchability was graded on 5 points arbitrary scale where 5 represents the best stretchable characteristics.
Sensory evaluation of the baking quality of cheese
About 100g of each shredded cheese was topped on unbaked pizza base separately, with tomato sauce, but without any vegetable fillings, and transferred to an oven maintained at 180-200°C. The pizza was baked for 20-25min to allow melting (till the shreds fused uniformly) of cheese. The baking characteristics of the cheese were assessed by the arbitrary numbers, where 5 points was awarded for the best pizza.
Sensory evaluation of cheese
The cheeses were organoleptically evaluated by semi-trained panelists, following the recommendations of IDF [38] The panelists evaluated cheese for appearance, flavor, texture, taste and overall sensory score) using a nine-point hedonic scale, with 1 being the worst, and 9 the best quality.
Statistical analysis
Collected data were analysed by GenStat Discovery Edition 3, GenStat Procedure Library Release PL15.2, Version 7.22 DE (Copyright 2008, VSN International Ltd) for Analysis of Variance (ANOVA). The significant for means was tested using LSD (Least Square Difference) method at 5% level of significance.
Results and Discussion
Numerical optimization for maximum Milk Clotting Activity (MCA)
The measured expansion of MCA varied from 17.23-65.36 Otani units (Supplementary information S1). Regression model fitted to experimental results of MCA (Supplementary information S2) showed that Model F-value of 55.51 was significant (P<0.05) whereas, lack-of-Fit F-value of 1.36 was not significant (P>0.05). The fit of the model was also expressed by the coefficient of determination R2=0.9804, indicating that 98.04% of the variability of the response could be explained by the model. The adjusted R2 of 0.9627 and adequate precision of 18.956 showed an adequate signal. Considering these criteria, the model (Equation 1) was selected for representing the variation of MCA and used for further analysis.
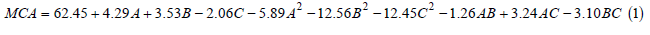
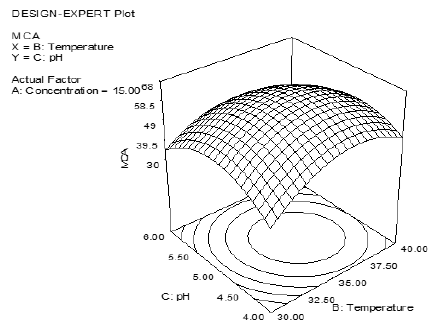
Figure 1: Response surface plot for MCA as a function of pH of milk and enzyme concentration at fixed temperature of 35°C.

Where A, B and C are the coded values of enzyme concentration,
temperature and pH of the milk respectively. In this case, A, B, C, A2,
B2, C2, AC and BC are significant model terms (P<0.05). The MCA in
above quadratic equation 1 had significant (P<0.05) positive linear
effect of enzyme concentration (A) and temperature of milk (B) at
95% confidence level. However, the linear term -pH of milk (C) had
a significant negative effect on MCA (P<0.05). The quadratic terms
of enzyme concentration (A) and temperature (B) and pH of milk
(C) had highly significant (P<0.05) negative effect on MCA. The
interaction term of enzyme concentration and pH of milk (AC) had
significant (P<0.05) positive effect on MCA. Other interaction terms
-temperature and pH of milk (BC) had a significant negative effect,
while interaction terms of enzyme concentration and temperature
of milk (AB) were found to be non-significant (P>0.05). An increase
in temperature of milk resulted in a higher MCA (Figure 1). The
activity reached a maximum when the temperature was at a certain
level, with non-significant improvement thereafter. Campos, et al.
[39] and Heimgartner, et al. [40] studied the effect of temperature
on the proteolytic activity and reported that proteolytic activity
increased with temperature to a maximum at 37°C. A possible
explanation for this phenomenon could be the denaturation of
proteins at higher temperature. The concentration of enzyme above
the optimum level had a negative effect on the activity of ginger
protease as shown in (Figure 1).
Amid, et al. [41] reported that increasing the buffer content
above the optimum volume caused a decrease in the total activity of
the extracted protease, which ultimately resulted in undue dilution
of solution. The pH of the milk has an important linear effect on
the final specific activity of the extracted enzyme. The maximum
MCA was observed at a pH of 5.0. Successful milk clotting agents at
optimum pH of substrate split the κ-casein at the bond between the
phenylalanine (105) and the methionine (106) residues diffusing
casino-peptide in the serum [42]. Below or above the optimum pH
had a negative effect on the total activity of milk (Figure 2). It may
be inferred that the large linear effect of pH on the final specific
activity of the enzyme extracted arises primarily from the change
in the intrinsic activity of the enzyme as a response to pH rather
than from changes in the extraction yield [43]. Mohamed [44]
observed the activity of Solanum dubium extract at pH 5.5. The
discrepancy may be due to the source of milk clotting enzymes and
the extraction method used.
Figure 2: Response surface plot for MCA as a function of pH and temperature at fixed enzyme concentration of 15μL/mL of milk.
Optimization
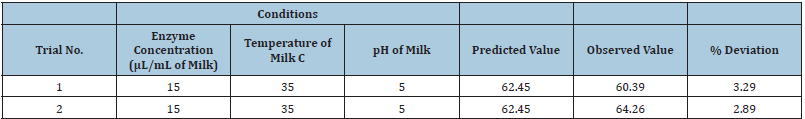
A numerical response optimization technique was applied to determine the optimum combination of enzyme concentration, temperature and pH of milk for the maximum MCA. Under the assumptions by a design expert, the optimum operating conditions for maximum MCA of milk were enzyme concentrations of 15μL/ mL of milk, pH 5.0 and milk temperature 35°C. The responses predicted by the software for these optimum process conditions reported MCA of 62.45 Otani units. (Table 1) shows the different conditions of the constraints for optimization.
Table 1: Different constraints for optimisation.

Verification of the model
Within the scope of the variables investigated in central composite rotatable design, additional experiments with different processing conditions were conducted to confirm the adequacy of the model equations. The conditions and the results of the confirmatory experiments are presented in (Table 2).
Table 2: Predicted and actual values of the responses at the optimized condition.
Physicochemical properties
Chemical composition: The chemical composition of the mozzarella cheeses made from Direct Acidification Rennet (DAR), Starter Culture Rennet (SCR), direct acidification ginger protease (DAGP) and Starter Culture Ginger Protease (SCGP) have been shown in (Table 3). It was observed from (Table 3) that there was a significant difference (P<0.05) in the moisture content of cheeses prepared from two coagulants. The moisture percentage in all cheeses are in line with the findings of Johnson, et al. [45] but lower than the findings of Nawaz, et al. [46]. The variation in results might be due to the difference in milk composition, the activity of the coagulant and processing techniques. The moisture contents in mozzarella cheese prepared using ginger protease were significantly (P<0.05) higher compared to rennet ones. Utsumi, et al. [47] explained that the molecular forces involved in the coagulation of casein by crude enzyme results in a greater water-binding capacity of the protein matrix. Another justification for more moisture retention might be due to the longer coagulation time for ginger protease as outlined by Johnson, et al. [45]. Regarding fat, (Table 3) revealed non-significant (P>0.05) difference among cheese samples. While the fat levels correlated well with the findings of Fasale, et al. [48], these were higher than reported by Sameen, et al. [49]. Cheese made using ginger protease showed comparatively lower fat content. Khan, et al. [50] revealed that plant protease take a longer time for coagulation compared to rennet and thus retain less fat in the final product.
Table 3: Chemical composition of mozzarella cheeses prepared from ginger protease and rennet.
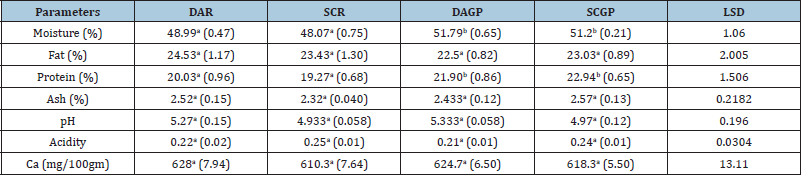
Values are the means of triplicates. Figures in the parentheses
represent the standard deviation. Values in the row bearing
similar superscript are not significantly different at 5% level of
significance. There was a significant difference (P<0.05) observed
in protein contents of cheeses prepared from two coagulants. The
protein in mozzarella cheeses made using ginger protease was
higher than that of rennet. The protein contents of DAR and SCR
are in line with the findings of Seth, et al. [51] but higher than the
findings of Sameen, et al. [49]. These disparities could be due to
the use of crude enzyme extract as it contains proteinous material
in it. Omueti and Jaiyeola [22] reported that an increase in protein
content might be due to retention of whey in the final cheese. It was
observed from (Table 3) that there was a non-significant (P>0.05)
difference in the ash among cheese samples. The ash contents of
mozzarella cheese are similar to the findings of Mijan, et al. [52];
Masud, et al. (1993) and Patel and Gupta (1986), who reported
ash contents in the range of 2.50 to 3.20%. (Table 3) also revealed
non-significant difference (P>0.05) in the pH of all cheese samples.
The results correlated with the findings of Mijan, et al. [52] and
Waheed, et al. [53] but lower than the reported values of Khan, et
al. [50]. The type of coagulating agent, processing technique and
source of milk might have caused such differences in results. (Table
3) also revealed a non-significant (P>0.05) effect on the acidity of
cheeses prepared by both coagulants. However, a slight increase
in acidity was observed in mozzarella cheese prepared with calf
rennet. The present findings follow a similar trend to Nunez, et al.
[54] who concluded higher acidity for animal rennet cheese in their
study was due to higher whey retention and subsequent lactose
fermentation.
The difference found in the calcium contents of the cheeses
produced by two coagulants was non-significant (P>0.05). The
values ranged from 610-628mg/100gm of cheese, which correlated
with the findings of Panthi [33]. Among four treatments, calcium
content was more in DAR followed by DAGP, SCGP and SCR.
According to Keller, et al. [55] calcium content inversely correlated
to the moisture content of the cheese. Similarly, Joshi, et al. [56]
reported that caseins are more hydrated as the level of bound
calcium decreases in milk. The higher calcium in cheese prepared
with direct acidification method (DAR and DAGP) may be due to
the effect of citric acid. Shakeel-Ur, et al. [57] reported citric acid
chelates more calcium than by any other acid.
Theoretical and actual yield: The theoretical and actual
yield of mozzarella cheeses prepared by using animal and plant coagulant by direct acidification and starter culture method have
been presented in (Figure 3). A non-significant difference was
observed in the theoretical yields of cheese samples prepared
from rennet and ginger protease. The findings are in accordance
with the results of Nawaz, et al. [46] but lower than the results of
Seth, et al. [51]. The slight variation in theoretical yields might be
due to the moisture contents in the final cheese [58]. Similarly, a
non-significant difference was observed in actual yields among all
treatments. Actual yields are similar to the findings of Zedan, et al.
[59] and, Bhattarai and Acharya [6].
Figure 3: Theoretical and actual yield of mozzarella cheeses.
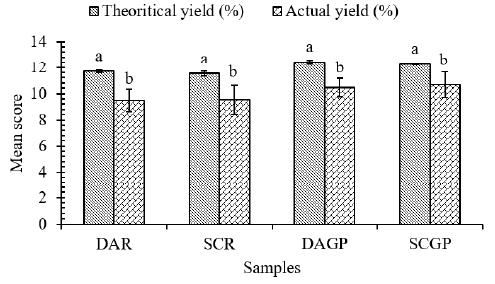
Figure 4: Meltability and stretchability of mozzarella cheeses.
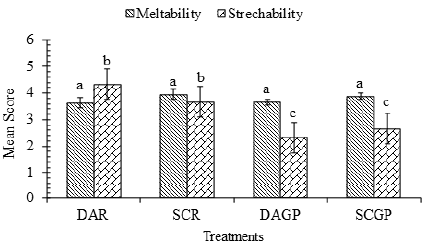
Meltability and stretchability: The meltability and stretchability of cheeses prepared using animal and plant coagulants by direct acidification and starter culture method are presented in (Figure 4). Analysis of variance of meltability revealed a non-significant (p>0.05) difference among all treatments. On 2nd day of storage, the cheese sample SCR (3.94±0.035) had highest meltability ratio followed by SCGP (3.89±0.015), DAGP (3.67±0.03) and DAR (3.64±0.04). The increased meltability in SCR and SCGP may be due to the decreased calcium content and higher amount of moisture present [60,61] compared to sample DAR and DAGP. Fresh cheese was typically firm and had poor melting properties although it was stretchable. However, as the cheese matures, the texture softens and there is an increase in the melt [62]. The improvement in meltability is due to dislodgement of para-casein matrix [63]. As time increased, the meltability of all cheese samples increased significantly, but the trend of increase remained the same Sameen, et al. [49]. The statistical analysis of stretchability showed samples were significantly different (p<0.05). The stretchability of sample DAR (4±0) was found to be highest followed by SCR (3.67±0.58), SCGP (3.89±0.015) and DAGP (2.33±0.58) as shown in (Figure 4). According to Joshi, et al. [56], a decrease in the calcium content would lead to the decreased structural rigidity of the cheese matrix consequently increasing the stretchability. Keller, et al. [55] and Oberg, et al. [64] reported an inverse relationship between stretchability and meltability of mozzarella cheese.
Sensory evaluation of cheese
The graphical representation of sensory scores of the cheese samples is shown in (Figure 5). The data regarding appearance showed a non-significant (p>0.05) difference among the samples. The average appearance value was comparatively higher for SCR (6±0.87) followed by SCGP (6.11±0.78), DAR (6±0.87) and DAGP (6±0.71). According to Delahunty, et al. [65], the appearance of cheese is a function of the interaction between cheese color and texture, and coagulant type used. Cheese made using ginger protease had significantly (p<0.05) higher score for flavor as compared to cheese made using rennet. The average flavor score was highest for DAGP (6.78±0.8) followed by SCGP (6.67±0.71), SCR (5.78±0.83) and DAR (5.67±0.71). This variation in cheese flavor might be attributed to the inherent property of the crude extract of ginger rhizome (an aromatic plant used as a spice) that provides a pleasant aroma and enhances the flavor of the cheese [66]. Chen, et al. [67] and Roseiro, et al. [10] reported that intense proteolytic action displayed by plant-coagulant enzyme enhances the flavor of the cheese. The data regarding taste, texture and overall acceptability showed a non-significant (P>0.05) difference among the samples. The average taste score was slightly higher for SCR (6.44±0.89) followed by DAR (6.22±0.97), SCGP (5.78±0.67) and DAGP (5.67±0.71). A mild bitter taste was observed in cheese made from plant coagulant, which may be the reason for low taste score in SCGP and DAGP. In a study by Dinakar, et al. [68], an aqueous extract of berries of Withania coagulans also showed a bitter taste in the final product This bitterness is associated with accumulation of the bitter peptides that contain more hydrophobic amino acid residues when coagulants from plant sources are used [69].
Figure 5: Graphical view of mean sensory scores of mozzarella cheeses.
Note: Values are the means of 9 panelists. Values on top of bars bearing similar superscript are not significantly different at 5% level of significance; where DAR, Direct Acidified Rennet; SCR, Starter Culture Rennet; DAGP, Direct acidified ginger protease and SCGP, Starter culture ginger protease.
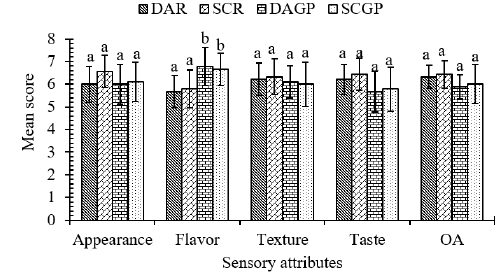
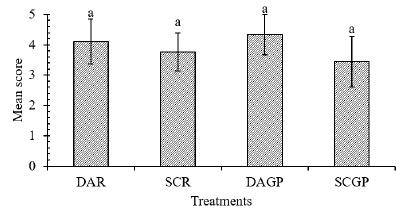
Figure 6: Graphical view of mean scores for baking quality of mozzarella cheeses.
Note: Values are the means of 9 panelists. Values on top of bars bearing similar superscript are not significantly different at 5% level of significance.
Conclusion
Protease enzyme was extracted from ginger (var. Nashe) rhizome by simple extraction method. The extracted enzyme displayed optimum milk clotting activity at pH5, temperature 35°C and enzyme concentration 15μL/mL of milk. The physico-chemical analysis, showed that fat, ash, acidity, pH, calcium and yield were similar (P>0.05) between cheeses prepared using ginger protease and calf rennet. Likewise, appearance, texture, taste and overall acceptance of the cheeses prepared using ginger protease were also similar (P>0.05) to that of control cheese prepared using calf rennet. Meltabilility and baking properties of the cheeses prepared using ginger protease were comparable to that of control cheeses. However lower stretchability was observed in cheese prepared using ginger protease. The results of this study revealed that physicochemical, functional and sensory attributes of mozzarella cheese prepared from ginger protease is comparable with the cheese made from calf rennet. This shows that the ginger enzyme is a potential substitute for rennet in mozzarella cheese preparation. Further studies using a pure enzyme from ginger are required to improve the potential use at commercial scales.
References
- Mistry VV, Andrson DL (1993) Composition and microstructure of commercial full fat and low fat cheese. Food Structure 12(2): 259-266.
- Vairo-cavalli S, Claver S, Priolo N, Natalucci C (2005) Extraction and partial characterization of a coagulant preparation from Silybum marianum flowers. Its action on bovine caseinate. Journal of Dairy Research 72(3): 271-275.
- Razzaq S (2003) Preparation of cottage cheese using sodom apple (Calotropis procera) latex as plant rennet. University of Arid Agriculture, Pakistan.
- Colavito AL (1997) High hill yak cheese production in Nepal: An analysis of privatisation policy incorporating in impacts of market for Agro‐industries in developing countries.
- Citro V (1981) A typical local product obtained from buffalo milk. Dairy-science-and-technique 32: 263-273.
- Bhattarai RR, Acharya PP (2010) Preparation and quality evaluation of mozzarella cheese from different milk sources. Journal of Food Science and Technology 6: 94-101.
- Ishak R, Idris YMA, Mustafa S, Sipat A, Muhammad SKS, et al. (2006) Factors affecting milk coagulating activities of Keisinai (Streblus asper) extract. International Journal of Dairy Science 1: 131-135.
- Kumar A, Grover S, Sharma J, Batish VK (2010) Chymosin and other milk coagulants: Sources and biotechnological interventions. Critical Reviews in Biotechnology 30(4): 243-258.
- Cavalcanti MTH, Teixeira MFS, Filho JLL, Porto ALF (2004) Partial purification of new milk-clotting enzyme produced by Nocardiopsis sp. Bioresource Technology 93(1): 29-35.
- Roseiro LB, Barbosa M, Ames JM, Wilbey RA (2003) Cheese making with vegetable coagulants- the use of Cynara L. for the production of ovine milk cheeses. International Journal of Dairy Technology 56(2): 76-85.
- Egitoa AS, Girardet JM, Laguna LE, Poirson C, Molle D, et al. (2007) Milk clotting activity of enzyme extract from sunflower and albizia seeds and specific hydrolysis of bovine κ-casein. International Dairy Journal 17(7): 816-825.
- Duarte AR, Duarte DMR, Moreira KA, Cavalcanti MTH, Filho JLL, et al. (2009) Jacaratia corumbensis O. Kuntze a new vegetable source for milk-clotting enzymes. Brazilian Archives of Biology and Technology 52(1): 1-9.
- Gomez R, Sanchez E, Vioque M, Ferreira J, Tejada L, et al. (2001) Microbiological characteristics of ewe’s milk cheese manufactured using aqueous extracts of flowers from various species of cardoon Cynara L. Milchwissenschaft 56 (1): 16-19.
- Liburdi K, Boselli C, Giangolini G, Amatiste S, Esti M (2019) An evaluation of the clotting properties of three plant rennets in the milks of different animal species. Foods 8(12): 600.
- Gomes S, Belo AT, Alvarenga N, Dias J, Lage P, et al. (2019) Characterisation of Cynara cardunculus L. flower from alentejo as coagulant agent for cheese making. International Dairy Journal 91: 178-184.
- Zikiou A, Zidoune MN (2019) Enzymatic extract from flowers of Algerian spontaneous Cynara cardunculus: Milk‐clotting properties and use in the manufacture of a Camembert‐type cheese. International Journal of Dairy Technology 72(1): 89-99.
- Pagthinathan M, Ghazali HM, Yazid AM, Foo HL (2019) Extraction, purification and characterisation of a milk-clotting protease from ‘kesinai’ (Streblus asper) leaves. International Food Research Journal 26(3): 913-922.
- Barbosa M, Corradini C, Battistotti B (1981) Cheese-making experiments carried out on some Italian cheeses with vegetable rennet from cardo (Cynara cardunculus L.). Dairy Science and Technique 32(4): 203-221.
- Fernandez-Salguero J, Sanjuan E, Montero E (1991) A preliminary study of the chemical composition of Guia cheese. Journal of Food Composition and Analysis 4(3): 262-269.
- Roseiro MLB (1991) Ewe’s milk cheese-making in Portugal using a vegetable rennet. Sheep Dairy News 8(2): 74-75.
- Mohamed MA, O’Connor CB (1996) Milk coagulation by Calotropis procera juice, effects of juice storage time and temperature trials for cheese making. Indian Journal of Dairy Science 49: 277-285.
- Omueti O, Jaiyeola O (2006) Effects of chemical and plant based coagulants on yield and some quality attributes of tofu. Nutrition and Food Science 36(3): 169-176.
- Choi KH, Laursen RA, Allen KN (1999) The 2.1 A structure of a cysteine protease with proline specificity from ginger rhizome, zingiber officinale. Biochemistry 38(36): 11624-11633.
- Ichikawa Y, Sasa H, Michi K (1973) Purification of ginger protease. Journal of Japan Society of Nutrition and Food Science 26: 337-383.
- Thompson EH, Wolf ID, Allen CE (1973) Ginger rhizome: A new source of proteolytic enzyme. Journal of Food Science 38(4): 652-655.
- Su HP, Huang M, Wang H (2009) Characterization of ginger proteases and their potential as a rennin replacement. Journal of the Science of Food and Agriculture 89(7): 1178-1185.
- Huang XW, Cen LJ, Luo YB, Guo HY, Ren FZ (2011) Purification, characterization and milk clotting properties of ginger protease. Journal of Dairy Science 94(5): 2259-2269.
- Caffini NO, Lopez LMI, Natalucci CL, Priolo NS (1988) Higher plant proteases I. general characteristics, physiological role and applications. Acta Farm Buenos Aires 7: 195-213.
- Horne DS, Banks JM (2004) Rennet induced coagulation of milk. In: Fox PF, McSweeney PLH, Cogan T Guinee T, (Eds.), Cheese: Chemistry, Physics and Microbiology, pp. 147-170.
- Faro CJ, Moir AJ, Pires EV (1992) Specificity of a milk clotting enzyme extracted from the thistle Cynara cardunculus L.: Action on oxidized insulin and k-casein. Biotechnology Letters 14: 841-846.
- Hailu Y, Seifu E, Yilma Z (2014) Clotting activity of camel milk using crude extracts of ginger (Zingiber officinale) rhizome. African Journal of Food Science and Technology 5(3): 90-95.
- Berridge NJ (1952) An improved method of observing the clotting of milk containing rennin. Journal of Dairy Research 19(3): 328-329.
- Panthi RR (2007) Preparation of mozzarella cheese from sour milk by direct acidification method. Food Technology Instruction Committee, Nepal.
- AOAC (1990) Official Methods of Analysis. (15th edn), Association of Official Analytical Chemist, USA.
- Pearson D (1981) The chemical analysis of food. (7th edn), UK, pp. 470-480.
- Vanslyke LL, Publow C (1910) The science and practice of cheese-making; A treatise on the manufacture of American Cheddar cheese and other varieties, intended as a text-book for the use of dairy teachers and students in classroom and workroom. Orange Judd Company, pp. 454-462.
- Muthukumarappan K, Wang YC, Gunasekaran S (1999) Short communication: Modified schreiber test for evaluation of mozzarella cheese meltability. Journal of Dairy Science 82(6): 1068-1071.
- IDF (1997) Sensory Evaluation of Dairy Products by Scoring. International Dairy Federation, Belgium.
- Campos R, Guerra R, Aguilar M, Ventura O, Camacho L (1990) Chemical characterization of proteases extracted from wild thistle (Cynara cardunculus). Food Chemistry 35(2): 89-97.
- Heimgartner U, Pietrzak M, Geerstsen R, Brodelius P, Fugueiredo SAC, et al. (1990) Purification and partial characterization of milk clotting proteases from flowers of Cynara cardunculus. Phytochemistry 29(5): 1405-1405.
- Amid M, Tan CP, Mirhosseini H, Aziz NA, Ling TC (2011) Optimisation of serine protease extraction from mango peel (Mangifera indica Chokanan). Food Chemistry 124(2): 666-671.
- Upadhyay KG (2003) Essential of cheese making. (1st edn), Gujarat Agricultural University, Anand Press, India, pp. 1-206.
- Sousa MJ, Malcata FX (1996) Effects of processing conditions on the caseinolytic activity of crude extracts of Cynara cardunculus L. Food Science and Technology International 2(4): 255-263.
- Abdalla MOM, Ali AAD, Mohamed EB (2010) Extraction, milk clotting activity, measurement and purification of Solanum dubium fresen (gubbain) for cheese making. World Journal of Dairy and Food sciences 5(2): 152-159.
- Johnson ME, Chen CM, Jaeggi JJ (2001) Effect of rennet coagulation time on composition, yield, and quality of reduced-fat cheddar cheese. Journal of Dairy Science 84(5): 1027-1033.
- Nawaz M, Masud T, Sammi S (2011) Quality evaluation of mozzarella cheese made from buffalo milk by using paneer booti (Withania coagulans) and calf rennet. International Journal of Dairy Technology 64(2): 218-226.
- Utsumi S, Kinsella JE (1985) Forces involved in soy protein gelation: Effects of various reagents on the formation, hardness and solubility of heat-induced gels made from 75, 11S and soy isolate. Journal of Food Science 50(5): 1278-1282.
- Fasale AB, Patil VS, Bornare DT (2017) Process optimization for mozzarella cheese from cow and buffalo milk. International Journal of Food Science and Technology 7(1): 165-173.
- Sameen A, Anjum FM, Huma N, Nawaz H (2008) Quality evaluation of mozzarella cheese from different milk sources. Pakistan Journal of Nutrition 7(6): 753-756.
- Khan RS, Masud T (2013) Comparison of buffalo cottage cheese made from aqueous extract of Withinia coagulans with commercial calf rennet. International Journal of Dairy Technology 66(3): 396-401.
- Seth K, Bajwa U (2015) Effect of acidulants on the recovery of milk constituents and quality of mozzarella processed cheese. Journal of Food Science and Technology 52(3): 1561-1569.
- Mijan MA, Haque MA, Habib MA, Wadud MA (2010) Evaluation of quality of mozzarella cheese. Bangladesh Veterinarian 27(1): 36-42.
- Waheed M, Hussain MB, Majeed M, Rehman TU, Khan MU (2017) Quality evaluation of cheese prepared by plant coagulants: Carica papaya and Moringa oleifera leaves. Russian Journal of Agricultural and Socio-Economic Sciences 4: 232-239.
- Nuñez M, Pozo BFD, Rodriguez-Marin MA, Gaya P, Medina M (1991) Effect of vegetable and animal rennet on chemical, microbiological, rheological and sensorial characteristics of La Serena cheeses. Journal of Dairy Research 58: 511-519.
- Keller B, Olson NF, Richardson T (1974) Mineral retention and rheological properties of mozzarella cheese made by direct acidification. Journal of Dairy Science 57(2): 174-180.
- Joshi NS, Muthukumarappan K, Dave RI (2004) Effect of calcium on microstructure and meltability of part skim mozzarella cheese. Journal of Dairy Science 87(7): 1975-1985.
- Shakeel R, Farkye N (2006) Effect of setting pH on the properties of mozzarella cheese made by direct acidification of whole milk standardized with dry milk protein concentration. Australian Journal of Dairy Technology 61(1): 8-12.
- Melilli C, Lynch JM, Carpino S, Barbano DM, Licitra G, et al. (2002) An empirical method for prediction of cheese yield. Journal of Dairy Science 85(10): 2699-2704.
- Zedan IA, Abou-Shaloue Z, Zaky SM (2014) Quality evaluation of mozzarella cheese from different milk types. Alexandria Science Exchange Journal 35: 162-177.
- McMahon DJ, Oberg C (1990) Influence of fat, moisture and salt on functional properties of mozzarella cheese. Australian Journal of Dairy Technology 53(2): 98-101.
- Fife RL, McMahon DJ, Oberg CJ (1996) Functionality of low fat mozzarella cheese. Journal of Dairy Science 79(11): 1903-1910.
- Rowney M, Roupas P, Hickey MW, Everett DW (1999) Factors affecting functionality of mozzarella cheese. Australian Journal of Dairy Technology 54(2): 94-102.
- Sheehan JJ, Guinee TP (2004) Effect of pH and calcium level on the biochemical, textural and functional properties of reduced-fat mozzarella cheese. International Dairy Journal 14(2): 161-172.
- Oberg CJ, Merrill RK, Moyes LV, Brown RJ, Richardson GH (1991) Effects of Lactobacillus helveticus culture on physical properties of mozzarella cheese. Journal of Dairy Science 74(12): 4101-4107.
- Delahunty CM, Drake MA (2004) Sensory character of cheese and its evaluation. In: Fox PF (Ed.), (3rd edn), Cheese: Chemistry, Physics and Microbiology, pp. 455-488.
- El-Aziz MA, Mohamed SHS, Seleet FL (2012) Production and evaluation of soft cheese fortified with ginger extract as a functional dairy food. Polish Journal of Food and Nutrition Sciences 62(2): 77-83.
- Chen S, Zhao J, Agboola S (2003) Isolation and partial characterization of rennet-like proteases from Australian cardoon (Cynara cardunculus L.). Journal of Agriculture and Food Chemistry 51(10): 3127-3134.
- Dinakar P, Mathur MP, Datta RD (1989) Differences in proteolytic behavior in cheddar cheese prepared with calf and vegetable rennet. Indian Journal of Dairy Science 42: 792-796.
- Singh TK, Drake MA, Cadwallader KR (2003) Flavor of cheddar cheese: A chemical and sensory perspective. Comprehensive Reviews in Food Science and Food Safety 2(4): 166-189.
- Bansal N, Drake MA, Piraino P, Broe ML, Harboe M, et al. (2009) Suitability of recombinant camel (Camelus dromedarius) chymosin as a coagulant for cheddar cheese. International Dairy Journal 19(9): 510-517.
- Fathollahi I, Hesari J, Azadmard S, Oustan S (2010) Influence of proteolysis and soluble calcium levels on textural changes in the interior and exterior of Iranian UF white cheese during ripening. World Academy of Science Engineering and Technology 4(6): 399-404.
- Barbano DM (1999) Controlling functionality of mozzarella cheese through process control. Proc Marschall Italian Cheese Sem, Rhone-Poulenc, USA.
- Rudan MA, Barbano DM, Yun JJ, Kindstedt PS (1999) Effect of fat reduction on chemical composition, proteolysis, functionality, and yield of mozzarella cheese. Journal of Dairy Science 82(4): 661-672.
© 2021 Adhikari BR. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






