- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Influence of Zinc and Ascaridia galli Infection on Morphometry of Intestine in Chickens
Levkut M Jr1, Revajová V1*, Dvorožňáková E2, Grešáková L3, Ševčíková Z1, Selecká E1, Karaffová V1, Levkutová M4 and Levkut MSr1,5
1Department of Morphological Disciplines, University of Veterinary Medicine and Pharmacy, Slovakia
2Institute of Parasitology, Slovak Academy of Sciences, Slovakia
3Institute of Animal Physiology, Centre of Bioscences SAS, Slovakia
4Department of Epizootology, Parasitology and Protection of One Health, University of Veterinary Medicine and Pharmacy, Slovakia
5Neuroimmunological Institute SAS, Slovakia
*Corresponding author: Revajová V, Department of Morphological Disciplines, University of Veterinary Medicine and Pharmacy, Slovakia
Submission: November 29, 2021;Published: December 20, 2021

ISSN: 2576-9162 Volume8 Issue5
Abstract
To obtain more information of zinc influence to nematode infection in intestine, we studied the effect of inorganic zinc and Ascaridia galli infection on morphometry of jejunum in chicken broilers. Thirty five day old chickens (n= 24), COBB 500 breed were included in a 14-day experiment. Chickens were divided into 4 groups of 6 chickens each: control (C), Ascaridia galli (AG), Zinc group (Zn), and combined group (AG+Zn). Samples from jejunum for determination of villus height, cutting surface area and depth of crypt were taken at 7 and 14 day during necropsy. Positive effect of zinc on the height of villi was observed at the second sampling in the combined group. Similarly, depth of crypts was significantly shorter in chickens of combined AG+Zn group in comparison to AG group. The ratio of villus height to depth of crypt in jejunum demonstrated significantly lower coefficient in Ascaridia galli infected group what supposed severe accumulation of immunocompetent cells and oedema in pure AG group. The data of our findings suggest that dietary supplementation of inorganic Zn into the diet of chickens improved small intestinal morphology in nematode infected broilers what can suggest better feed conversion in Ascaridia galli infected intestine.
Keywords: Morphology; Crypts; Villi; Inorganic zinc; Nematode; Poultry
Introduction
Ascaridia spp. are the most common and important worms in poultry, and infection can produce a variety of clinical signs including intestinal blockage, diarrhoea and even death of affected avian species [1]. Gradual obtaining of resistance to the deworming drugs forces the researcher to find alternative strategies for treating of parasite infections in poultry production. The life cycle of Ascaridia galli is direct and birds get infection by ingestion of embryonated eggs [2]. Ingested, embryonated eggs are transported to the duodenum and/or jejunoileum. In these segments of intestine they hatch and larvae are released within 24 h [3]. The embedded larvae induced changes in the mucosal thickness as reduced villi height at 7, 10, 14, 21, and 28 dpi and influenced the degree of general cellular infiltration in the lamina propria of the mucosal layer [2]. Two weeks after infection, lesions such as the formation of lymphoid aggregations and mild lymphocytic infiltration were determined in the jejunal lamina propria of inoculated birds [4]. Zinc supplementation decreases oxidative stress markers and increases phagocytosis. Moreover, Zn promotes monocyte adhesion to endothelial cells in vitro what is important for the production of pro-inflammatory cytokines [5]. There is several studies followed beneficial effect of zinc on some immunological parameters of nematode infected intestine in chickens. On the other hand, no information is available in the literature regarding the effects of zinc source focusing to the morphometry of intestine infected nematode in broilers. To obtain more information of zinc influence to nematode infection in intestine, we studied the effect of inorganic zinc and Ascaridia galli infection on morphometry of jejunum in chicken broilers.
Material and Methods
Experimental design
Thirty five day old chickens (n=24), COBB 500 breed were included in a 14-day experiment. Chickens were randomly divided into 4 groups of 6 chickens each (n=6): control (C), Ascaridia Galli (AG), Zinc group (Zn), and combined group (AG+Zn). The infected groups (AG; AG+Zn) were inoculated orally with 500 embryonated A. galli eggs in 0.5ml of Phosphate Buffered Saline (PBS) per bird at day 2 of experiment. The infection was carried out using a plastic Pasteur pipette inserted via the oesophagus to the level of the crop. Inorganic form of zinc was administered daily per os to Zn and AG+Zn groups from day 2 to 13 day of experiment. At the end of experiment the chickens were first anesthetised using intra-abdominal injection of xylazine (Rometar 2%, SPOFA, Czech Republic) and ketamine (Narkamon 5%, SPOFA, Czech Republic) at doses of 0.7ml/kg body weight and then were exsanguinated. The broilers were sacrificed by decapitation. After sacrifice, the abdominal cavity was opened and whole small intestine was open to check the changes on mucous membrane and presence of parasites. Samples from mid part of jejunum in 3cm length were taken. All procedures were carried out in accordance with the European Community guidelines (EU Directive 2010/63/EU) for the care and use of animals for scientific purposes.
Infective material and inoculation
Ascaridia galli eggs were obtained from adult female worms from the intestines of naturally infected chickens. The eggs were isolated from the female worm uterus according to Permin A et al. [6] by a gentle mechanical maceration in 0.5 N NaOH. The eggs were embryonated in 0.1 N NaOH for 4 weeks at 28 °C in the dark. During incubation the egg suspensions were oxygenated three times per week by stirring. Eggs embryonation was evaluated microscopically on weekly basis starting from day 14. Finally, the embryonated A. galli eggs were stored in 0.1 N NaOH at 4-6 °C and regularly oxygenated until the infection of experimental animals. The infected groups were inoculated orally with 500 embryonated A. galli eggs in 0.5ml of Phosphate Buffered Saline (PBS) per bird. Aqueous solution of inorganic form of zinc, 0.5ml/50mg ZnSO4 (Lachema Brno, ČR) was administered daily per os to Zn and AG+Zn groups from 2 to 13 days of the experiment.
Sample collection
Samples from intestine for determination of villus length, cutting surface area and depth of crypth were taken at 7 and 14 day during necropsy.
Intestinal histomorphology
Jejunum samples were fixed in 10% neutral buffered formalin and prepared using paraffin embedding techniques. Three consecutive sections (5μm) from each jejunum were stained using haematoxylin and eosin and observed for histomorphology. The villus height and its surface area (from the tip of the villus to the crypt junction), crypt depth, villus height-crypt depth ratio were measured from 70 to 100 randomly selected villi with one section per chicken at 100× magnification. The morphometry was evaluated by using the NIS-Elements Advanced Research Programme (Ver. 3.00, Nikon, Japan).
Statistical analysis
Statistical analysis was done using one-way ANOVA with the post hoc Tukey multiple comparison test (GraphPad Prism 5.0, San Diego, CA, USA) to determine significant differences between experimental groups. For the ratio of depth crypts to height of villi structures was used the Dunnett’s test, in order to compare experimental group again control group. The values in the graphs are presented as means ± Standard Deviation (SD), and p-values <0.05 were considered statistically significant.
Results
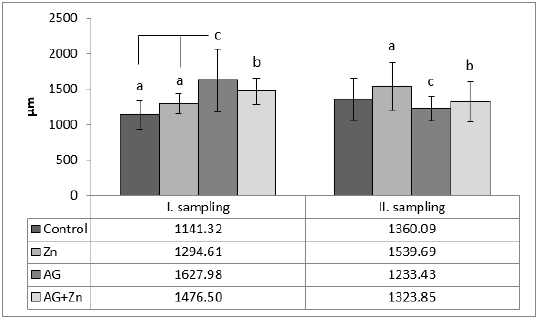
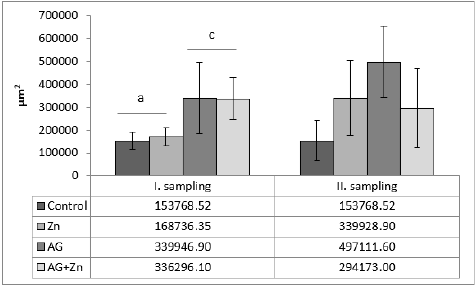
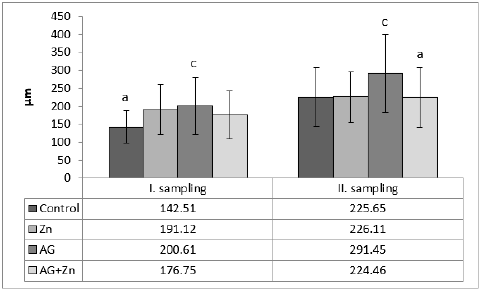
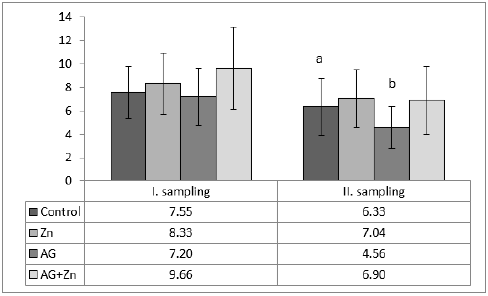
Morphometry of villi showed increase of height of villi in AG+Zn group to controls (p < 0.05) and in Ag group to Zn chickens (p < 0.001) in the first sampling (Graph 1). On the other hand, second sampling demonstrated increase of villi in Zn group to AG+Zn group (p < 0.05) and Zinc group to AG group (p < 0.001). Cutting surface of villi showed significant changes only in the first sampling (Graph 2). Differences were found in AG+Zn and AG group to controls (p < 0.001). Similarly, cutting surface was changed in AG+Zn and AG group to zinc group (p < 0.001). Depth of crypts demonstrated changes in AG group to controls (p < 0.001) in the first sampling and in AG group to AG+Zn chickens (p < 0.001) in the second sampling (Graph 3). Ratio of height of villi to depth of crypt in each group showed any significant changes at first sampling. Second sampling demonstrated decreased ratio height of villi versus depth of crypts in AG group (p < 0.05) comparing to control (Graph 4). The most of larvae were found deeply in the jejunal mucosa, i. e. in the lumen of the crypts (Figure 1) or in the lamina propria of the villi (Figure 2) during first sampling.
Figure 1: Larvae of ascaridia galli in jejunal crypt (white arrow) 7 days after infection (H&E, bar = 20μm).
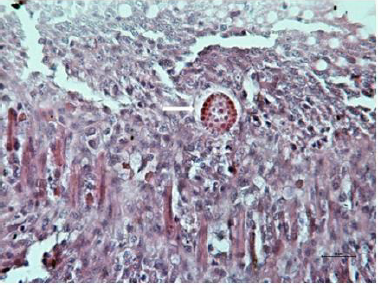
Figure 2: Larvae of ascaridia galli in jejunal lamina propria (white arrow) 7 days after infection (H&E, bar=10μm).

Graph 1: Height of villi in jejunum (abp < 0.05; acp < 0.001).
Graph 2: The cutting surface of villi structures in jejunum (acp < 0.001).
Graph 3: The depth of crypts in jejunum (acp < 0.001).
Graph 4: The ratio of height of villi to depth of crypts structures in jejunum (abp < 0.05).
Histological examination showed the similar density of A. galli larvae in both AG and AG+Zn groups. Also size of larvae was unchanged in A. galli infected groups.
Discussion
The morphology of small intestine is considered as the main
indicator of normal gut histology [7]. A part of the functional status
of the small intestine is defined by villus height and crypt depth
[7]. The measurements of villus height and crypt depth provide an
indication of the maturity and functional capacity of small intestinal
enterocytes [8]. Various substances and also different physiological
or pathological conditions including lesions induced by parasites
may change the villus height and crypt depth.
Our current results demonstrated that infection of Ascaridia
galli extended height of villi in first sampling. On the other hand,
seven days dietary supplementation of zinc did not influence height
of villi in this period. However, second sampling showed positive
effect of zinc on growth of jejunal villi and differences between other
groups were lower as in first sampling. Similarly, cutting surface of
villi was increased in infected groups and later also in zinc group.
Positive effect of inorganic zinc on growth of villi demonstrated
Levkut M et al. [9] in previous experiment. Shao Y et al. [10] found in
intestine of chickens after dietary supplementation of zinc repaired
intestinal injury by reducing the apoptotic index of ileal epithelial
cells, enhancing villus height and villus-height/crypt-depth ratio.
Finally, better feed conversion after dietary supplementation of
zinc demonstrated De Grande A et al. [11]. An increased villus
height is related to an increased digestion and absorption of
nutrient [12]. Collet SR [13] supposed that intestinal surface is
directly proportional to digestive and absorptive efficiency and can
influence feed conversion. Taking this into account, improved villus
and crypt morphology after supplementation of dietary zinc could
have positive impact on intestinal health and growth performance
in Ascaridia galli infected broilers in our experiment. Similarly,
our data showed that administration of dietary zinc influenced
height of villi and positively changed ratio of height to depth of
crypt what can suggest better feed conversion in parasite infected
intestine. Previously presented evaluation of metallothionein in
intestinal tract [9] in current trial showed higher values in zinc
group what was connected with administration of inorganic zinc.
Tran CD et al. [14] found shorter villi in duodenum and jejunum
of metallothionein (MT) -/- mice fed 15mg Zn and/or 50mg Zn
compared with MT +/+ mice. Authors concluded that presence of
MT may enhance morphological and functional development of
the gut. Our unpublished results are consistent with study Tran CD
et al. [14] showing increased level of MT to better ratio of villus
height: crypt depth.
In contrast, Zn deficiency has been shown to cause villus atrophy,
elevated levels of mucosal cell apoptosis, ulceration, inflammation
as well as reduction in crypt proliferation [11]. Considering the role
of zinc as a coenzyme for more than 300 enzymes particularly in
RNA-DNA synthesis and cell proliferation [15], these enhancements
in intestine morphology may be explained by the beneficial effects
of zinc on cell proliferation and differentiation.
Changes in depth of crypts with coefficient ratio villus
height: crypt depth were demonstrated mainly in second
sampling. Decreased ratio of height of villi to depth of crypts
was demonstrated in Ascaridia galli infected group at 14 day of
infection. Luna-Olivares LA et al. [2] observed reduced height of villi
at 7 and 14 day of experiment in infected group. In our experiment
height of villi increased at these days of experiment. We suggest
that increase of villi in AG group in our experiment was caused
by oedema and infiltration with immunocompetent inflammatory
cells. Our consumption is supported by coefficient of ratio villusdepth
of crypt which demonstrated the lowest ratio number in AG
group. This number shows an inflammatory process in crypt part of
jejunum. This is consistent with increase of eosinophils, heterophils,
CD4+, CD8+ and IgM+ lymphocytes in crypts and villi previously
reported from this experiment [16]. Similarly, increased cutting
surface of villi in infected groups corresponds with inflammatory
signs in first sampling of our experiment [17,18]. This experiment
also showed that administration of inorganic zinc did not influence
the density and size of larvae. On the other hand, our previously
published results from this experiment demonstrated beneficial
effect on immunological parameters.
Conclusion
Positive effect of zinc on height of villi was followed at the second sampling in combined AG+Zn group. Similarly, depth of crypts was shorter in AG+Zn group. Ratio of height of villi to depth of crypt demonstrated the significantly decreased coefficient in Ascaridia galli infected group what supposed severe accumulation of immunocompetent cells and oedema in AG group. The data of these findings suggest that dietary supplementation of Zn into the normal diet of chickens improved small intestinal morphology in nematode infected broilers what can suggest better feed conversion in Ascaridia galli infected intestine.
Acknowledgement
This research was funded by Slovak Research and Developmental Agency APVV-15-0165, and Grant Agency for Science of Slovakia VEGA 1/0355/19, 1/0107/21.
References
- Anand K, Wakode S (2017) Development of drugs based on benzimidazole heterocycle: Recent advancement and insights. Int J Chem Stud 5(2): 350-362.
- Luna-Olivares LA, Kyvsgaard NCH, Ferdushy T, Nejsum P, Thamsborg SM, et al. (2015) The jejunal cellular responses in chickens infected with a single dose of ascaridia galli Parasitol Res 114(7): 2507-2515.
- Hansen M, Terhaar C, Turner D (1956) Importance of the egg shell of ascaridia galli to the infectivity of its larva. J Parasitol 42(2): 122-125.
- Schwarz A, Gauly M, Abel H, Daş G, Humburg J, et al. (2011) Immunopathogenesis of ascaridia galli infection in layer chicken. Dev Comp Immunol 35(7): 774-784.
- Bonaventura P, Benedetti G, Albarède F, Miossec P (2015) Zinc and its role in immunity and inflammation. Autoimmun Rev 14(4): 277-285.
- Permin A, Pearman M, Nansen P, Frandsen F, Pearman M (1997) An investigation on different media for embryonation of ascaridia galli Helminthologia 34(2): 75-79.
- Laudalio V, Passantino L, Perillo A, Lopresti G, Passantino A, et al. (2012) Productive performance and histological features of intestinal mucosa of broiler chickens fed different dietary protein levels. Poult Sci 91(1): 265-270.
- Seyyedin S, Nazem MN (2017) Histomorphometric study of the effect of methionine on small intestine parameters in rat: an applied histologic study. Folia Morphol (Warsz) 76(4): 620-629.
- Levkut M, Fukasová M, Bobíková K, Levkutová M, Čobanová K, et al. (2017) The effect of inorganic or organic zinc on the morphology of the intestine in broiler chickens. Fol Vet 61(3): 52-56.
- Shao Y, Lei Z, Yuan J, Yang Y, Guo Y, et al. (2014) Effect of zinc on growth performance, gut morphometry, and cecal microbial community in broilers challenged with Salmonella enterica serovar typhymurium. J Microbiol 52(12): 1002-1011.
- De Grande A, Leleu S, Delezie E, Rapp Ch, De Smet S, et al. (2020) Dietary zinc source impacts intestinal morphology and oxidative stress in young broilers. Poult Sci 99(1): 441-453.
- Awad WA, Hess C, Hess M (2017) Enteric pathogens and their toxins-induced disruption of the intestinal barrier through alteration of tight junctions in chickens. Toxins (Basel) 9(2): 60.
- Collet SR (2012) Nutrition and wet litter problems in poultry. Anim Feed Sci Tech 173(1-2): 65-75.
- Tran CD, Cool J, Xian CJ (2011) Dietary zinc and metallothinein on small intestinal disaccharidases activity in mice. World J Gastroenterol 17(3): 354-360.
- Frassinetti S, Bronzetti G, Caltavutoro L, Cini M, Croce CD (2006) The role of zinc in life: A review. J Environ Pathol Toxicol Oncol 25(3): 597-610.
- Karaffová V, Revajová V, Dvorožňáková E, Grešáková Ľ, Levkut M, et al. (2021) Effect of inorganic zinc on selected immune parameters in chicken blood and jejunum after galli infection. Agriculture 11(6): 551.
- Duff M, Ettarh RR (2002) Crypth cell proliferation rate in small intestine of the zinc-supplemented mouse. Cell Tissues Organs 172(1): 21-28.
- Luna-Olivares LA, Ferdushy T, Kyvsgaard NC, Nejsum P, Thamsborg SM, et al. (2012) Localization of ascaridia galli larvae in the jejunum of chickens 3 days post infection. Vet Parasitol 185(2-4): 186-193.
© 2021 Revajová V. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






