- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Antibacterial Activity of Cranberry (Vaccinium Macrocarpon) Pomace in Relation to its Enzymatic Hydrolysis
Junqiao Wang1,2, He Gao1,2, Xiahua Yin1, Steve W Cui1, Moussa Diarra1 and Qi Wang1*
1Guelph Research and Development Center, Agriculture and Agri-Food Canada, Guelph, Ontario, Canada
2State Key Laboratory of Food Science and Technology, China-Canada Joint Lab of Food Science and Technology (Nanchang), Nanchang University, China
*Corresponding author:Qi Wang, Guelph Research and Development Center, Agriculture and Agri-Food Canada, Guelph, Ontario, Canada
Submission: August 08, 2021;Published: September 15, 2021

ISSN: 2576-9162 Volume8 Issue4
Abstract
As a natural antibacterial product, cranberry phenolic extracts have been used to control the presence of pathogenic bacteria in poultry. Cranberry pomace, a by-product of cranberry processing, is a potential source of phenolic compounds. However, most phenolic compounds are bonded with cell wall substances and not readily extractable, hence, not bioavailable. The objective of the current study was to explore the potential of enhancing the antimicrobial activity of cranberry pomace by enzymatic hydrolysis of cell wall matrix. A blend of pectinase and cellulase-hemicellulase was applied to the cranberry pomace; three types of extraction solvents of different polarity were used to isolate the phenolic compounds and the antibacterial activities of the extracts against three common pathogenic bacterial strains, i.e., E. coil ATCC 25922, S. Heidelberg, and S. Enteritidis, were determined by the agar diffusion assay. The enzyme treatment significantly elevated the recovery rate of total phenolic compounds irrespective of extraction conditions, however, it also compromised the recovery of anthocyanidins and anthocyanidins, which could negatively impact the antibacterial activity. Among the three extraction methods tested, the methanol/water/acetic acid (85:15:0.5, v/v/v) was found to be the most favorable method to extract the antibacterial phenolic compounds. These results indicated that the combination of enzymatic treatment and methanol/water/acetic acid (85:15:0.5, v/v/v) extraction was a potential method to increase the antibacterial constituents in cranberry pomace. Further study is necessary to select specific enzymes to minimize the degradation of phenolic compounds while releasing them from the cell wall matrix.
Keywords:Cranberry pomace; Phenolic compounds; Anti-bacterial activity
Introduction
Cranberry (Vaccinium macrocarpon), one of the native fruits in North America, contains a wide spectrum of bioactive substances that are beneficial to human and animal health [1,2]. They have long history of use in prevention and treatment of bacterial infections of the urinary tract and infections of gastric mucosa [3,4,5]. Abundant evidences have indicated that the antibacterial function of various berries, including cranberry, is mainly attributed to their phenolic compounds [6,7]. Recently, cranberry product have been tested to control the presence of pathogenic bacteria in food animal production due to their significant antimicrobial activity against several food borne pathogens. In a previous study, we reported that supplementation of a commercial cranberry fruit extract reduced the populations of Enterococcus spp. in cecal and cloacal samples and lowered the mortality rate of chicken compared to a non-supplemented control diet [8]. In another study, we revealed that the antibacterial action of cranberry press cake extracts was associated with the disruption of cell wall biosynthesis of bacteria pathogens [9].
Cranberry pomace is the by-product of cranberry processing industry comprising primarily fruit seeds, skins and stems, which is a potential source of phenolic compounds. However, a large proportion of phenolics in cranberry pomace (> 75%) belongs to insoluble bound phenolics, which form covalent bonds with cell wall molecules including pectin, cellulose, hemicellulose and structural proteins [10]. These bound phenolics are not readily extractable, thus have little or no antimicrobial function. Aiming to improve the antimicrobial activity, Vattem DA et al. [11] applied a solid-state bioprocess using the fungus Rhizopus oligosporus to cranberry pomace; the fermentation resulted in an increase in the total extractable phenolic content which correlated well with the increase in antimicrobial activity. Enzymatic hydrolysis was also exploited to improve the extraction of bound phenolic compounds from bilberry [12] and unripe apples [13]. The main component of cell wall of cranberry are cellulose, pectin and some hemicelluloses [14]. Thus, aside from cellulase and pectinase, a large group of carbohydrases, such as hemicellulases, exoglucanase, β-glucosidase etc. are required to completely degrade cell wall carbohydrates, leading to liberation of insoluble-bound phenolics [15]. The results from previous studies showed the increment of extractable phenolic compounds due to disruption of the cell wall matrix by enzymatic hydrolysis, however, the effect of enzymatic hydrolysis on the antibacterial activity remains to be investigated. Therefore, in the current study, we treated the cranberry pomace with a blend of carbohydrate hydrolases to maximize the disintegration of the cell wall matrix and liberating the bound phenolics. The objective was to investigate the effects of enzyme hydrolysis on the antibacterial activity of cranberry pomace in relation to their phenolic profiles.
Materials and Methods
Enzymatic hydrolysis
Organic frozen cranberry was purchased from Fruit d’Or (Québec, Canada) from which freeze dried cranberry pomace was produced as previously described [16]. The pomace sample was ground with a commercial coffee blender into a fine powder and stored at -20 °C before use. For enzyme hydrolysis, the ground pomace sample was suspended in 50mM sodium acetate buffer (pH 5.0) containing a mixture of enzymes including Viscozyme®L (Sigma, USA, a cellulolytic enzyme mixture from Aspergillus sp., containing a wide range of carbohydrases, including, cellulase, β-glucanase, arabinase, xylanase and other hemicellulases) and pectinase (from aspergillus niger, Sigma, USA), incubated at 37 °C under constant stirring for 24h. Upon completion, the hydrolysate was placed in a 105 °C oil bath and heated for 5 min to inactivate the enzymes. The mixture was then freeze-dried and stored at -20 °C prior to further tests.
Extraction scheme for phenolic compounds
Three types of solvents with different polarity were used to extract phenolic compounds from cranberry pomace before or after enzymatic treatment, respectively. They are water/methanol (85:15, v/v) (WM) (Samples 1,4), acetone/methanol/water (40:40:20, v/v/v) (AMW) (Samples 2,5) and methanol/water/ acetic acid (85:15:0.5, v/v/v) (MWA) (Samples 3,6). These extracts were concentrated by rotary evaporation under reduced pressure at 40 °C and then freeze-dried to obtain the dry samples.
Purification of extracts
Sample 6 was further purified using reversed-phase Sep-Pak C18 Solid Phase Extraction (SPE) cartridge (Millipore Waters Chromatography, Milford, USA, Lot No. W2281C1) to remove undesired polar components such as proteins or sugars. The SPE cartridge was first pre-conditioned with 20mL of 100% methanol and equilibrated with 20mL of water. Then 1mL of Sample 6 was slowly loaded onto the cartridge and washed by water until the phenol-sulfuric acid reaction showing negative. All the water elutes were collected and freeze-dried (Sample 9). The phenolic fraction was collected by eluting with 40% methanol. The eluent was first evaporated using rotary evaporation at 40 °C under vacuum and then freeze-dried (Sample 7). The purified phenolic extracts (Sample 7) and the polar constituents (Sample 9) were stored at -20 °C until further analysis and uses in antimicrobial activity studies.
Identification and quantification of phenolics
Total phenolic content determination: The Total Phenolic Content (TPC) was determined by a colorimetric reaction using the Folin-Ciocalteu method [17] and the Total Phenolic Content (TPC) was expressed as milligrams of gallic acid equivalent per gram of dry matter (GAe/g DM). Briefly, 25μL of gallic acid standard or samples were mixed with 125μL of 0.2M Folin-Ciocalteu reagent in a 96-well microplate and reacted for 10min. Then, a saturated sodium carbonate solution (125μL) was added and the mixture was reacted for 30min prior to detecting at 765nm using a visible-UV microplate kinetic reader (EL 340, Bio-Tek Instruments, USA).
Anthocyanin composition analysis: Samples 3,6 and 7 were used for anthocyanin composition analysis. Samples were dissolved in 85% methanol (v/v) and filtered through a 0.2μm PTFE membrane filter (VWR, Mississauga, ON, Canada) prior to analysis. LC-MS/MS analysis was performed using a Thermo® Scientific Q-Exactive™ Orbitrap mass spectrometer equipped with a Vanquish™ Flex Binary UPLC System (Waltham, MA, USA). Kinetex XB-C18 100A column (100 x 4.6mm, 2.6μm, Phenomenex Inc., Torrance, CA, USA) was used. The binary mobile phase consisted of solvent A (99.9% H2O/ 0.1% formic acid) and solvent B (94.9% MeOH/ 5% ACN/ 0.1% formic acid). The following solvent gradient was used: 0-40min, 0% to 80% B; 40-42min, 80% to 100% B; 42-44min, 100% B; 44-44.5min, 100% to 0% B; 44.5-51min, 0% B. The column temperature was set at 25 °C, the flow rate was set at 0.7mL/min, and the injection volume was 5μL; peaks were monitored at 520nm. Positive ionization mode was used; the spray voltage was set at 3.5kV. Mass spectrometry data was collected using Full-MS/DDMS2 (TopN=5) method, with NCE set at 30 and intensity threshold set at 3.9e5 counts. Data was visualized and analyzed using Thermo FreeStyle™ 1.4 software.
Anthocyanidin composition analysis: The anthocyanidin composition analysis was conducted using an UHPLC system (Thermo Fisher Scientific, San Jose, CA) linked to a diode array detector and a Chrom Quest software for data processing. An aliquot (5mL) of extracts were hydrolyzed by adding 3.3mL of 2 M HCl and incubated at 100 °C for 1h and filtered through 0.45μm PVDF filter prior to injection to the UHPLC. Analytical separation of anthocyanidin was carried out on a Phenomenex C18 column (3μm, 4.6×150mm, Phenomenex Inc., Torrance, CA, USA) with a gradient elution procedure at a flow rate of 0.7mL/min. The mobile phase consisted of 10% formic acid aqueous solution (solvent A) and 100% methanol (solvent B). The elution profile was as follows: linear gradient from 95% A to 40% A, 0-20min; isocratic elution 40% A, 20-25min; gradient decrease from 40% A to 0% A, 25-30min. The column temperature was set at 25 °C. Diode array detection was set at 520nm and peak identification in cranberry samples was carried out by comparing retention time and spectra of unknown peaks with reference standards (delphinidin chloride, cyaniding chloride, pelargonidin chloride, peonidin chloride, petunidin chloride, and malvidin chloride). The concentration of anthocyanidin was expressed as milligrams of anthocyanidin per gram of cranberry sample dry weight.
Antimicrobial activity test
Bacterial strains: The extracts were individually tested against a panel of microorganisms, including Escherichia coli (E. coil) ATCC 25922, Salmonella Heidelberg (S. Heidelberg) and Salmonella Enteritidis (S. Enteritidis). Bacterial strains were cultured overnight at 37 °C in Mueller Hinton Agar (MHA). Viable Bacterial Counts (CFU) were determined on SENSITITRE Nephelometer according to 0.5 McFarland standards.
Antimicrobial test: Using a sterilized swab, around one colony of bacteria was added to 5mL of sterilized water, the CFU was adjusted using SENSITITRE Nephelometer with 0.5 McFarland standards and diluted 50 times using sterilized saline solution or Muller Hinton Broth (MHB). The diluted bacteria were mixed well and coated onto the surface of MHA in a sterile petri plate. Four wells were cut from each agar plate using tips of sterilized glass pipette and 50μL of extract solution was loaded onto each well. After incubation face upwards for 20h at 37 °C, all plates were examined for any zones of growth inhibition. All inhibitory activity assay were performed in duplicate.
Statistical analysis
Experimental values are expressed as the mean ± standard error. The results were analyzed using one-way analysis of variance (ANOVA) followed by Turkey’s tests with p=0.05. Statistical significance was determined by SSPS 26 program (SSPS Inc., Chicago, IL, USA).
Results
Yields and total phenolic contents of extracts
The three types of solvents used in this study have different polarity and thus primarily extract different classes of phenolic compounds from cranberry pomace [18]. Specifically, the solvent of WM mainly extracts the most water soluble phenolic compounds, the AMW extracts the most apolar phenolic compounds, and MWA mainly extracts anthocyanins [19]. The yields and total phenolic contents of the three extracts before or after enzymatic treatments of the pomace were summarized in Table 1. For samples without enzymatic treatment, AMW extraction (Sample 2) recovered the most total phenolic compounds (12.8mg GAe/g DW), followed by MWA extraction (10.5mg GAe/g DW, sample 3,) and WM extraction (5.9g GAe/g DW, Sample 1). After enzymatic treatment, the extraction yields from all the three extraction schemes were remarkably increased from below 20% to over 50%, although the concentration of total phenolic compounds in these extracts were reduced. Nevertheless, the recovery of total phenolic compounds from all the extractions are evidently increased after enzymatic treatment. Based on the dry weight of pomace, the TPC recoveries from AMW (16.9mg GAe/g) and MWA extraction (17.1mg GAe/g) were similar which were obviously higher than the WM extraction (10.2mg GAe/g). This result indicated that enzymatic treatment liberated some of the bound phenolic compounds from the cranberry pomace.
Table 1: Yield and Total Phenolic Content (TPC) of the extracts from cranberry pomace before or after enzyme-treatment by three extraction methods.

a. The content was calculated based on the dry weight of the extracts, (mg GAE/g ).
b. The content was calculated based on the dry weight of the pomace, (mg GAE/g).
Sample 6 was further purified by solid phase extraction, during which, one phenolic fraction was recovered in 40% methanol (Sample 7) at a yield of 1.9% based on the dry weight of Sample 6; the other fraction (Sample 9) was recovered in water at a yield of 63%. Since Sample 6 and Sample 7 each contained 29.7 and 403 mg GAe/g of total phenolic compound respectively (Table 1), this data indicates that approximately 26% of phenolic compounds in Sample 6 were recovered in Sample 7 and 51% of that in Sample 9.
Composition analysis of phenolic compounds and monosaccharides
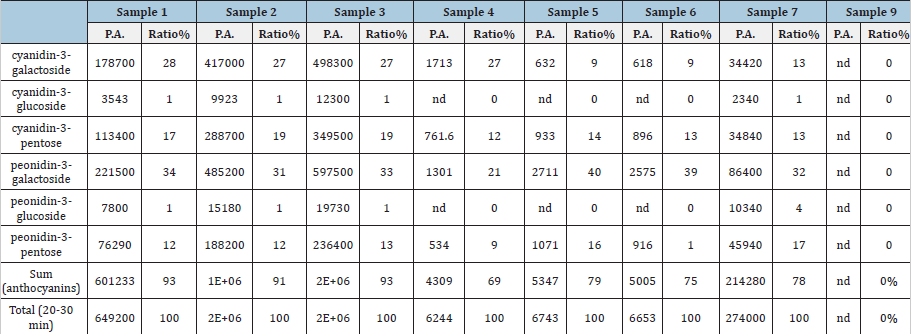
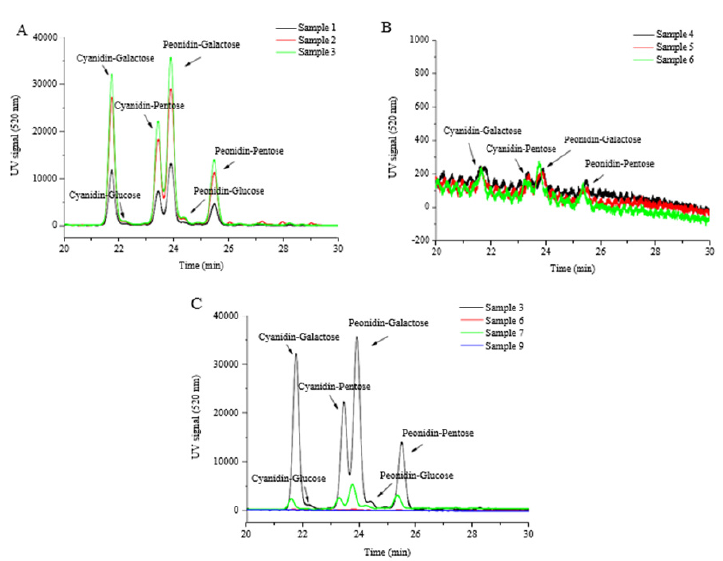
Anthocyanin composition: Aanthocyanins are a group of major phenolic compounds existing in cranberry pomace. In terms of chemical structure, anthocyanin is anthocyanidin linked with one or more sugar moieties. A total of 13 compounds were detected by HPLC at 520nm from different extracts of cranberry pomace (Table 2). Among these compounds, 7 anthocyanins were identified according to the respective standards, including cyanidin-3-pentose, peonidin-3-pentose, cyanidin-3-galactoside, cyanidin-3-glucoside, peonidin-3-galactoside, peonidin-3- glucoside, and delphinidin-3-galactoside. By calculating the peak area, the predominate anthocyanins found in the extracts from cranberry pomace without enzymatic hydrolysis are cyanidin-3- galactoside, cyanidin-3-pentose, peonidin-3-pentose, and peonidin- 3-galactoside (Table 3 and Figure 1A). This data were consistent with the previous reports [16,20]. Enzymatic treatment of pomace decreased the anthocyanin content in the extracts (Figures 1B). The relative proportions of the 4 major anthocyanins in the pomace were altered by enzyme treatment, although they were still the major anthocyanin constituents in the enzyme treated extracts. It was observed that the decomposition of cyanidin-3-galactoside was greater than that of peonidin-3-galactoside and peonidin-3- pentose, which is in line with a previous report [21].
Table 2: Identification of anthocyanins in various cranberry pomace extracts.
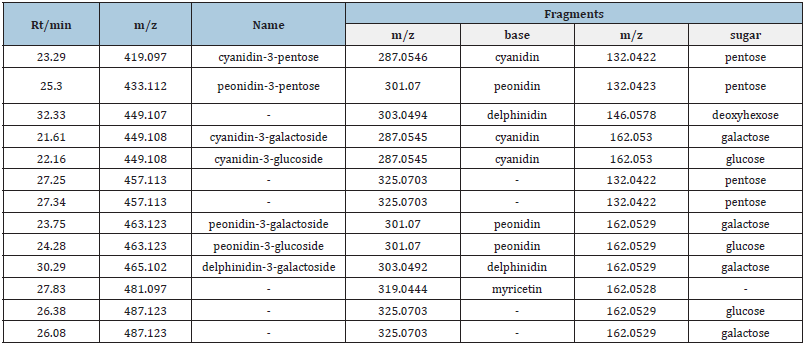
Table 3:Relative contents of anthocyanins in cranberry pomace extracts obtained from different extraction methods.
Note: P.A. means peak area detected in 520nm; nd means non-detected.
Note: nd means not detected.
Figure 1: HPLC profile of anthocyanins in different fractions of cranberry pomace. A. Samples 1-3, extracted from un-treated pomace; B. Samples 4-6, extracted from enzyme-treated pomace; C. Samples 7, 9, fractionated from Sample 6.
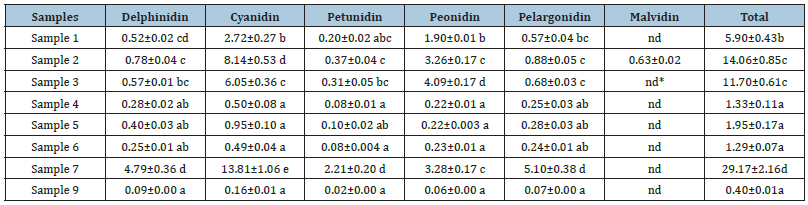
Anthocyanidin composition: The composition of anthocyanidins in cranberry pomace extracts were shown in Table 4 and Figure 2. Samples 1-3, the extracts from the cranberry pomace without enzyme treatment, were mainly composed of two types of anthocyanidins, i.e. cyanidin and peonidin, which is in agreement with the data reported in literatures [22]. Among the three extracts, malvidin was detected only in Sample 2 and cyanidin content was also notably higher in Sample 2 compared to the rest extracts. Consequently, Sample 2 had the highest anthocyanidin content, which obviously contributed to its highest TPC among all the extracts.
Table 4:Anthocyanidin contents of different fractions extracted from cranberry pomace (mg/g). *not detected.
Means (n= 3) with different letters in the same row are significantly different (P < 0.05).
Figure 2:HPLC profiles of anthocyanidins in different extracts from cranberry pomace. A. Samples 1 - 3, extracted from un-treated pomace; B. Samples 4 - 6, extracted from enzyme-treated pomace; C. Samples 7, 9, fractionated from Sample 6.
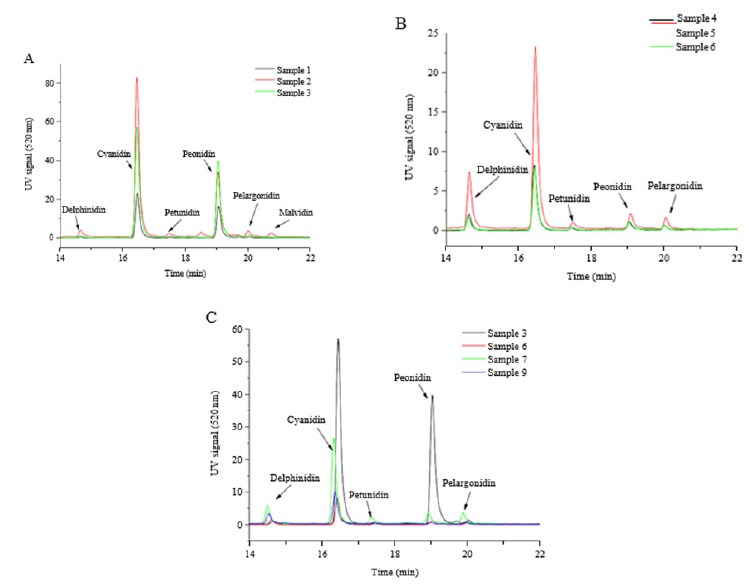
The extracts, Samples 4 - 6, obtained from enzyme treated cranberry pomace contained significantly lower levels of anthocyanidins compared to those from un-treated pomace, which was consistent with the decreasing trend in TPC in these extracts. In particular, peonidin content decreased the most compared to other anthocyanidins, thus, the relative proportions of each anthocyanidin were altered. Among the three enzyme treated extracts, Sample 5 tended to have higher total anthocyanidin content owing to its higher contents of delphinidin and cyanidin, although statistically the difference was not significant. The rest anthocyanidin contents were almost identical among Samples 4-6. Taking into account of the extraction yield, the total anthocyanidin recovery after enzyme treatment was also significantly reduced (Table 5). Fractionation of Sample 6 greatly enriched the anthocyanidins in Sample 7. Around 43% of anthocyanidins in Sample 6 was recovered in Sample 7, whereas only 5.1% of that was recovered in Sample 9. Sample 6 and Sample 7 exhibited similar anthocyanidin composition profiles in which cyanidin was the dominant anthocyanidin.
Table 5: The recovery of anthocyanidins by different extraction methods from cranberry pomace (μg/g of DW)a.
a. the content was calculated based on the dry weight of the pomace, (μg/g of DW). Means (n= 3) with different letters in the same row are significantly different (P < 0.05)
Monosaccharide composition: The monosaccharide compositions in the pomace extracts were determined in order to understand the effectiveness of the enzymes used in this study on degrading the cell wall materials. As can be seen in Table 6, the contents of arabinose and galactose in enzyme treated extracts were largely elevated compared with the untreated extracts. The increase in these two types of monosaccharides are mostly resulted from pectinase-catalyzed degradation of pectins, the structure of which are mainly homogalacturonan, arabinogalactan and pectic galactan [23]. It appeared that a portion of associated anthocyanins, particularly, the galactosides and arabinosides of cyanidin and peonidin, were liberated and subsequently degraded by some glycosidase activities contained in the cellulase-himecellulase blend. A previous study has reported that β‐galactosidase was able to degrade cranberry anthocyanins to anthocyanidins and sugars [21]. It was noticed that rhamnose, xylose and mannose detected in the enzyme-treated pomace extracts (Samples 4-6) were absent in the un-treated extracts (Samples 1-3). These monosaccharides are mostly resulted from degradation of cell wall matrix by the hemicellulases in the enzyme blend used in this study.
Table 6: The monosaccharide contents in different extracts obtained from cranberry pomace (mg/g).
Antimicrobial activity
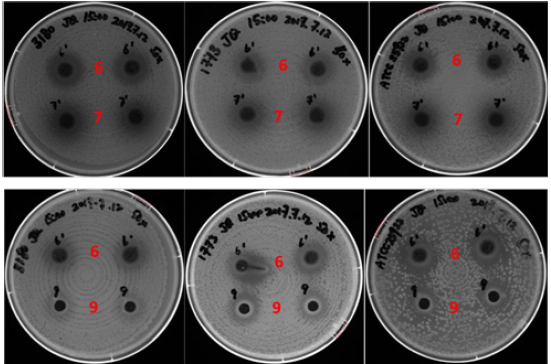
Figure 3:(A) Test of inhibition of the un-treated and enzyme-treated cranberry pomace extracts on S. Heidelberg (top row, 3180), S. Enteritidis (middle row 1773) and E. coli ATCC 25922 (bottom row). (B) Inhibitory test of the positive control FC111 (+) and enzyme-treated cranberry pomace extracts. Numerical numbers on the plates correspond respectively to the sample number in Table 1, i.e. “1” represents Sample 1.
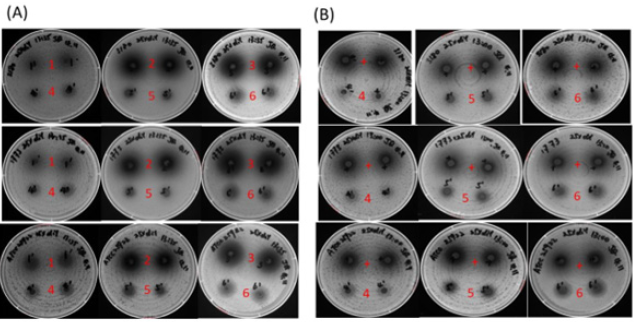
Inhibition effects of pomace extracts: All the extracts (Samples 1-6) exhibited inhibitory effect against the three bacteria strains tested, as evidenced by the clear inhibition zones in Figure 3. Generally speaking, at a given concentration, the larger the inhibition area, the stronger the antibacterial function is. The three bacteria strains showed similar sensitivity to each of the extracts. At the concentration of 25mg/well, the AMW extract Sample 2 and MWA extract Sample 3 from un-treated pomace showed a comparable inhibitory effect, whereas the WM extract Sample 1 showed a relatively weaker inhibition effect (top rows of each plate in Figure 3(A)). The inhibitory effect of the extracts coincided with their TPC, where Sample 1 had much lower TPC compared to Samples 2 and 3. At the same concentration of 25mg/well, the inhibitory effects of all extracts from enzyme-treated pomace (Samples 4-6) were significantly weaker than the respective counterparts from un-treated pomaces (bottom rows of each plate in Figure 3(A). Among the three extracts from enzyme treated pomaces, Sample 6 exhibited the most potent inhibitory activity against all the three bacterial strains although the total phenolic content and recovery rate were the same as of Sample 5. This result indicates that MWA was the most effect extraction solvent in order to isolate the antibacterial constituents from enzyme treated cranberry pomace.
In order to further evaluate the antimicrobial effects of the extracts after enzyme treatment, a comparative experiment was carried out between these extracts and a positive control (FC111) that showed significant antibacterial effect in our previous report [9]. As shown in Figure 3(B), the inhibitory effect of Samples 4 - 6 on E. coil ATCC 25922, S. Heidelberg and S. Enteritidis were relatively weaker compared to the positive control at a concentration of 25mg/well. Again, Sample 6 showed the highest inhibitory effect among the three extracts. Considering the total phenolic content of sample FC111 was 54mg GAe/g [16] verses 29.7 mg GAe/g in Sample 6, it appeared Sample 6 had a comparable antibacterial activity towards the tested strains.
Antibacterial function of extract fractions: The two fractions isolated from Sample 6 by reversed-phase solid phase extraction showed very different antibacterial activity. As shown in Figure 4, Sample 7 at the concentration of 1.25mg/well, containing 0.5mg GAe/well TPC, showed a comparable antimicrobial effect to Sample 6 at 25mg/well, containing 0.75mg GAe/well TPC. In a separate test, we also found that Sample 6 showed no inhibitory effect on E. coil ATCC 25922, S. Heidelberg and S. Enteritidis at 7mg/well (equivalent to 0.21mg GAe/well TPC). This result suggest that sample 7, the methanol eluate fraction from Sample 6, exhibited stronger antimicrobial activity than Sample 6. On the other hand, the water eluate fraction (Sample 9) also exhibited some inhibitory effect on the tested strains, but the effect was not as potent as with Sample 6 at the same concentration.
Figure 4: Comparison of inhibitory effects between Sample 6 extracted from enzyme-treated cranberry pomace and its two fractions Sample 6 and 9 against S. Heidelberg (left column), S. Enteritidis (middle column) and E. coli ATCC 25922 (right column). Numerical numbers refer to the sample name. The dosage for Samples 6, 7 and 9 were 25 mg, 1.25mg and 25mg per well respectively.
Discussion
In the current study, the AMW extract had much higher total phenolic content than the MWA and WM extracts, which was different from the results reported by Cote J et al. [7]. In that study, the same extraction methods were applied to cranberry fruits. Their result showed the AMW and MWA extracts had similar total phenolic contents, whereas WM extract had considerably higher total phenolic content compared to the result in the current study.
This observation indicates that the extractable phenolic compounds in the cranberry fruit is altered during processing. Firstly, during juice processing, many water-soluble phenolic compounds such as low-molecular-weight phenolic acids and anthocyanins were removed from the pomace. This could be accounted for the relative lower total phenolic contents in the WM extracts and MWA extracts. Secondly, the juicing process could have caused degradation of some proanthocynidins to small molecules that became extractable, hence, resulted in the highest anthocyanidin content in the AMW extract from pomace before enzyme treatment.
The carbohydrases blend applied in the current study effectively disintegrated the cell wall matrix of cranberry pomace, resulting increased monosaccharides contents in the extracts. In the meantime, the destruction of the cell wall matrix facilitated the liberation of the bound phenolic compounds that led to increased level of extractable total phenolic compounds irrespective the extraction scheme employed. Among the three extraction schemes, the AMW and MWA extracts had similar levels of total phenolic content that was also higher than WM extract.
In the current study, all the pomace extracts demonstrated inhibitory function towards the growth of the bacterial strains of E. coil ATCC 25922, S. Heidelberg and S. Enteritidis. The AMW and MWA extracts showed stronger antibacterial activity than WM extract, which is contradictory to the results reported by Cote J et al. [7]. In their study, the WM extract demonstrated the strongest antibacterial activity against E. coil ATCC 25922 and S. Typhimurium compared with the AMW and MWA extracts. This discrepancy could be attributed to the fact that their WM extract were produced from cranberry fruit and thus had higher total phenolic content as discussed earlier. Therefore, the WM extract from the cranberry fruit showed stronger antibacterial activity than the WM extract from cranberry pomace.
The extracts (Samples 4-6) from enzyme treated pomace showed lower antibacterial activity compared to their respective counterparts from un-treated pomace extracts (Figure 3A). The reduction of antibacterial activity can be partially explained by the fact that Samples 4-6 had lower TPC compared respectively to Samples 1-3, leading to weakened inhibitory effects at the same dosage. The lower TPC was partially caused by the dilution effect of carbohydrates that were co-extracted in the Samples 4-6 as indicated by the increase in monosaccharide contents in the extracts (Table 6). After enzyme treatment, some cell wall polysaccharides were hydrolyzed to oligo- or monosaccharides that could be solubilized together with the bound phenolic compounds, which increased the yield of extraction, but lowered the relative concentration of phenolic compounds in the extracts (Table 1). This was confirmed by purification of Sample 6 after which Sample 7 demonstrated potent antibacterial activity owing to removing the water soluble carbohydrates (Figure 4). Compared to Sample 5, Sample 6 had similar total phenolic content and anthocyanins and anthocyanidins profiles, however, it demonstrated stronger antibacterial activity against tested three bacterial strains. This could be explained by the contribution of other minor phenolic compounds in the extracts other than anthocyanins and anthocyanidins. Taken together, the methanol/water/acetic acid (85:15:0.5, v/v/v) proved to be the best the extraction scheme for accumulating antimicrobial substrates from enzyme treated cranberry pomace. Although the enzyme treatment in this study elevated the recovery of total phenolic compounds, it also evidently caused degradation of anthocyanins and anthocyanidin, which are known to be the major contributors to the antibacterial function of cranberry [7,24]. This factor could have also contributed to the reduction of antibacterial activity in the enzyme treated extracts. Therefore, further study is required to carefully select cell wall disintegrating enzymes that are able to effectively disrupt cell wall matrices while minimize the degradation of phenolic compounds. This can be achieved by identifying the glycosidase activities that led to degradation of the specific phenolic compounds and eliminating the enzymes of concern.
Conclusion
In conclusion, the current study demonstrated that phenolic extracts from cranberry pomace could effectively inhibit the growth of pathogenic E. coil ATCC 25922, S. Heidelberg, and S. Enteritidis. The enzymatic treatment by pectinase and a blend of cellulase and hemicellulases caused disruption of the cell wall matrix of cranberry pomace and increased the total extractable phenolic compounds, hence, its potential antibacterial activity. However, certain glycosidase activities contained in the hemicellulases resulted in degradation of anthocyanins and anthocyanidins which could negatively impact the antibacterial activity of the cranberry pomace and its phenolic extracts. Methanol/water/acetic acid (85:15:0.5, v/v/v) proved to be the best the extraction scheme for accumulating antimicrobial substrates from enzymatic treated cranberry pomace among the three extraction schemes tested. More research is needed to develop specific enzyme blends that could effectively enrich the extractable phenolic compounds in the cranberry pomace to enhance its antibacterial functions.
Acknowledgement
The authors wish to thank assistances from Ronghua Liu for anthocyanidin analysis, Lili Mats and Honghui Zhu for anthocyanin analysis, Yolanda Brummer for monosaccharide composition analysis, and Qian Guo for technical assistants. The project was funded by Agriculture and Agri-Food Canada project ID J-002173 under Organic Cluster III.
References
- Neto CC (2007) Cranberry and its phytochemicals: A review of in vitro anticancer studies. J Nutr 137(1): 186S-193S.
- Ruel G, Couillard C (2007) Evidences of the cardioprotective potential of fruits: The case of cranberries. Mol Nutr Food Res 51(6): 692-701.
- Blumberg JB, Camesano TA, Cassidy A, Kris-Etherton P, Howell A, et al. (2013) Cranberries and their bioactive constituents in human health. Adv Nutr 4(6): 618-632.
- Côté J, Caillet S, Doyon G, Sylvain JF, Lacroix M (2010) Bioactive compounds in cranberries and their biological properties. Crit Rev Food Sci Nutr 50(7): 666-679.
- Howell AB (2007) Bioactive compounds in cranberries and their role in prevention of urinary tract infections. Mol Nutr Food Res 51(6): 732-737.
- Conner D (1993) Naturally occurring compounds: Antimicrobial in foods. Marcel Dekkar Inc, New York, USA.
- Côté J, Caillet S, Doyon G, Dussault D, Sylvain JF, et al. (2011) Antimicrobial effect of cranberry juice and extracts. Food Control 22(8): 1413-1418.
- Leusink G, Rempel H, Skura B, Berkyto M, White W, et al. (2010) Growth performance, meat quality, and gut microflora of broiler chickens fed with cranberry extract. Poult Sci 89(7): 1514-1523.
- Diarra MS, Block G, Rempel H, Oomah BD, Harrison J, et al. (2013) In vitro and in vivo antibacterial activities of cranberry press cake extracts alone or in combination with β-lactams against Staphylococcus aureus. BMC Complement Altern Med 13(1): 90.
- White BL, Howard LR, Prior RL (2010) Release of bound procyanidins from cranberry pomace by alkaline hydrolysis. J Agri Food Chem 58(13): 7572-7579.
- Vattem DA, Lin YT, Labbe RG, Shetty K (2004) Antimicrobial activity against select food-borne pathogens by phenolic antioxidants enriched in cranberry pomace by solid-state bioprocessing using the food grade fungus Rhizopus oligosporus. Food Biotechnol Process Biochem 39(12): 1939-1946.
- Puupponen-Pimiä R, Nohynek L, Ammann S, Oksman-Caldentey KM, Buchert J (2008) Enzyme-assisted processing increases antimicrobial and antioxidant activity of bilberry. J Agric Food Chem 56(3): 681-688.
- Zheng H, Hwang IW, Chung SK (2009) Enhancing polyphenol extraction from unripe apples by carbohydrate-hydrolyzing enzymes. J Zhejiang Univer Sci B 10(12): 912-919.
- Holmes AB, Rha C (1978) Structure and chemical composition of cranberry cell wall material. J Food Sci 43(1): 112-115.
- Shahidi F, Yeo J (2016) Insoluble-bound phenolics in food. Molecules 21(9): 1216.
- Ross KA, Ehret D, Godfrey D, Fukumoto L, Diarra M (2017) Characterization of pilot scale processed Canadian organic cranberry (Vaccinium macrocarpon) and blueberry (Vaccinium angustifolium) juice pressing residues and phenolic-enriched extractives. Intern J Fruit Sci 17(2): 202-232.
- Singleton VL, Rossi JA (1965) Colorimetry of total phenolics with phosphomolybdic-phosphotungstic acid reagents. Am J Enology Viticul 16(3): 144-158.
- Caillet S, Lorenzo G, Côté J, Doyon G, Sylvain JF, et al. (2012) Cancer chemopreventive effect of fractions from cranberry products. Food Res Intern 45(1): 320-330.
- Wu X, Prior RL (2005) Systematic identification and characterization of anthocyanins by HPLC-ESI-MS/MS in common foods in the United States: Fruits and berries. J Agric Food Chem 53(7): 2589-2599.
- Macheix JJ, Fleuriet A, Billot J (1990) Fruit phenolics. (1st edn), CRC Press, Boca Raton, USA.
- Wightman JD, Wrolstad RE (1995) Anthocyanin analysis as a measure of glycosidase activity in enzymes for juice processing. J Food Sci 60(4): 862-867.
- Watson D, Bushway A, Bushway R (2004) Separation of peonidin and cyanidin, two anthocyanidins, in cranberries by capillary electrophoresis. J Liq Chromatogr Relat Technol 27(1): 113-121.
- Andreani ES, Karboune S, Liu L (2021) Structural characterization of pectic polysaccharides in the cell wall of stevens variety cranberry using highly specific pectin-hydrolyzing enzymes. Polymers 13(11): 1842.
- Lacombe A, McGivney C, Tadepalli S, Sun X, Wu VCH (2013) The effect of American cranberry (Vaccinium macrocarpon) constituents on the growth inhibition, membrane integrity, and injury of Escherichia coli O157:H7 and Listeria monocytogenes in comparison to Lactobacillus rhamnosus. Food Micro 34: 352-359.
© 2021 Qi Wang. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






