- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Evaluation of One Immunochromographic Method for Identification Human Blood Meal Sources in Vectors of Chagas Disease
Zumaquero Rios JL1*, Sarracent Perez J1, Sandoval Ruiz C A1 and Ramos Ligonio A2
1Parasites and vectors laboratory, Biological Science Faculty, Benemerita Universidad Autonoma of Puebla Mexico
2Angel Ramos Ligonio Laboratory of Immunology and Molecular Biology Chemical Science Faculty of Chemical Sciences, Veracruz University, Mexico
*Corresponding author: Zumaquero Rios JL, Parasites and vectors laboratory, Biological Science Faculty, Benemerita Universidad Autonoma of Puebla Mexico
Submission: March 31, 2021;Published: April 21, 2021

ISSN: 2576-9162 Volume8 Issue3
Introduction
Diseases transmitted by vectors have increased in recent years due to climate change and other reasons, they are the cause of millions of deaths worldwide [1]. Trypanosoma cruzi is the etiological agent of Chagas disease. It is transmitted by blood-sucking insects belonging to the subfamily Triatominae, which is distributed in the American continent and includes 141 species in 15 genera [2]. These vectors vary in their role in the transmission of Chagas disease to humans, due to their preference for ecotypes, wild or domestic. The cycle of T cruzi has been considered an important problem to establish and design control and surveillance strategies [3]. In this sense the determination of the feeding source in insects (blood-meal sources) is essential to identify sources of infection and risk factors involved in the transmission cycles [4]. In triatomines, the methods used for this purpose include serological and others based on molecular techniques. The serological methods, (the most used in the literature is the ELISA) consume a lot of time because they require the preparation of specific antiserum for each possible vertebrate host [5]. One of the molecular approaches that have proved to be useful and potent is the heteroduplex assay of the mitochondrial cytochrome B gene (cyt b) [6]. This technique can detect differences in the DNA sequences of this gene between species due to differences in the electrophoretic profiles. More recently, other methods have been published for the same purpose. High Resolution Melt (HRM) of the cyt b gene [7], proteomics-based approaches using liquid chromatography in tandem with mass spectrometry to identify the peptides of the hemoglobin specific to the host [8], a combination of two molecular techniques, cloning and q PCR, from DNA obtained from the abdomen of the vector [9], sequence analysis of two heminated PCR, followed by Restriction Fragment Length Polymorphism (RFLP) [10].All these methods require the collection of vectors and taking them to the laboratory for subsequent analysis. In this study, we validated an immunochromatographic assay, to determine, using the dejections of the vector, if it has fed on human blood. The method was compared with the heteroduplex assay of the mitochondrial cytochrome B gene (cyt b). The results show that the test is specific and sensitive; it can determine the human feeding source up to 62 days after the event, at that time with the same sensitivity and specificity as the heteroduplex. The concordance index calculated by the coefficient κ was almost perfect for the samples from vectors fed with human blood between the two methods at both, 32 and 62 days.
Materials and Methods
This study was approved by the bioethics commission of the Hospital of the Benemérita Autonomous University of Puebla, Mexico.
Study groups
We used specimens from a triatomine colony established in the laboratory of parasitology and vectors of the Faculty of Biological Sciences of the Autonomous University of Puebla, which were kept in black plastic containers at a temperature of 27±2 °C and relative humidity (HR) of 70% ± 2, optimal for the development in laboratory conditions of these species (Martinez- Ibarra et al, 2012) A total of 210 adult specimens of the species Meccus pallidipennis Stål were selected and 7 groups were made (30 in each group) or categories according to the feeding sources, which were formed as follows: 1-human/ rabbit, 2-human /chicken 3-rabbit /chicken 4-chicken/ human 5-human, 6-chicken and 7-rabbit. The combinations of the categories were based on the criteria of Schofield (1994) [11] it recognizes that triatomines can have different sources of food at each stage of their development.
Human blood was obtained from 10 volunteers, which allowed periodic feeding with pathogen-free insects. The volunteers were asked for informed consent and a protocol review was carried out by the ethics committee of the University Hospital of Puebla, according to the standards for handling human samples of the Helsinki Code and the World Health Organization (WHO). The pathogen-free rabbits and chickens were obtained from the Bioterium of the Benemérita Autonomous University of Puebla (BUAP), “Bernard Claude”. The experiments with animals were approved by the Committee of Ethics, Management and Care of laboratory animals of the BUAP, according to the Mexican regulation for these purposes (NOM-062-ZOO-1999). For groups 1,2,3, and 4, the feeding at time 0 was with the blood of the species that appear first, followed by a feeding every 15 days with the blood of the species after mentioned, until completing 5, for a total of 60 days of experimentation. Group 5 received throughout the experiment feeding on human blood, 6 with chicken blood and 7 with rabbit blood.
Determination of human hemoglobin in triatomine dejections
Under the biweekly diet, the triatomines were fed, according to the groups and blood type. The dejections of the specimens were collected individually at 32 and 62 days with 1ml pipettes in 1.5mL eppendorf vials, the volume of the dejections were completed at 310μL with phosphate-buffered saline pH 7.2 (PBS) and homogenized with the purpose of immunological (immunochromatographic assay) and molecular (heteroduplex) assay
A immunochromatographic assay
The SUMASOHF rapid test (International TecnoSUMA immunoassay center, Havana Cuba) is used for the clinical determination of occult blood in human feces. It is an immunoassay sandwich used for the diagnosis of gastrointestinal diseases that cause bleeding. It is using monoclonal and polyclonal antibodies for the detection of human hemoglobin, with good sensitivity, positive results can be observed immediately. The sample flows through the absorbent device, the conjugate formed by a monoclonal antihuman hemoglobin / colloidal gold antibody migrates through a chromatographic process to the region of the sample where it will bind to another anti-human hemoglobin monoclonal antibody and this reaction produces a purple-pink band. In the absence of human hemoglobin, no band will be produced in the positive reagent zone. The results are read after 10 minutes, as recommended by the manufacturer. The sensitivity is 100% and its specificity for human hemoglobin has been verified with turkey, pig, bovine, equine, pigeon, chicken, goat and rabbit hemoglobin. For the determination of human hemoglobin in the stool, a volume of 100μL of the sample was used.
b- immunochromatographic assay with dry stool
After feeding between days 30, to 40 days, dried triatomine dejection was collected from the different groups, (5 samples from each group, 35 total samples), 50mg were weighed individually in eppendorf vials and dissolved in 200μL of PBS pH 7.2 for the immunoassay.
c-heteroduplex
The same dejections from the vector, were used for the extraction of DNA and the immunochromatographic assay.
PCR was performed for the amplification of specific cytochrome b fragments using the primers ctb1 and ctb2 [6]. The heteroduplex profiles were generated with Sus scrofa domesticus (pork) cytochrome b PCR products as a hybridization conductor and analyzed by electrophoresis in a 10% polyacrylamide gels in Tris borate-EDTA buffer (Bosseno et al, 2008,) [12]. The heteroduplex profiles of the samples were compared with those obtained from DNA from Homo sapiens (human), Gallus gallus domesticus (chicken) and Orcytolagus cuniculus (rabbit). Strict protocols and controls were used in all PCR assays to prevent and detect contamination. DNA extraction, amplification and product analysis were performed in separate laboratory areas dedicated to these purposes. Negative water control was included in each set of extraction and PCR reactions as contamination control. To evaluate the concordance between the two techniques when determining whether the vectors had been fed with human blood at the selected times, 32 and 62 days, we used the kappa index (κ), which represents the proportion of agreements observed beyond the chance [13,14].
Experiments with adult specimens captured in natural conditions in peri and intrahuman domicile
150 adults M. mazzotti specimens captured by workers and entomologists in the Sanitary Jurisdiction of the State of Oaxaca and 200 specimens of M. pallidipennis, adults, collected in southern areas of the state of Puebla, were kept in plastic containers with corrugated paper to simulate the habits of the organisms. All the specimens were fed with rabbit blood, 5 days after their capture and 15 days later. After the second feeding, the dejections were taken for studies with the immunochromatographic assay.
Discussion
The immunochromatographic assays for fecal occult blood detection are well accepted in laboratories and in the literature as a method for the detection of human hemoglobin [15]. Several studies have shown its usefulness in the detection of adenomas and colon cancer, only exceeded in sensitivity and specificity by the stool DNA test, but with the advantage of its low cost [16-18]. We selected the SUMASOHF test for our study because of its adequate sensitivity, it is capable of specifically detecting human hemoglobin at low concentrations, 10μg per gram of stool. In the total samples of the dejections of triatomine fed with human blood, their presence was detected at 32 days, not at 62 days, which is probably, because at this time the concentration of hemoglobin is below the sensitivity of the test. This problem could be solved with an immunoassay with a higher level of sensitivity [19]. In the heteroduplex in the cases of vectors fed with human blood at 32 days and 62 days, we found some cases where detecting the presence of human blood was practically impossible, especially in the case that the vectors had fed on other species after feeding with human hemoglobin, a situation that had already been reported by other authors. When the insects have fed on more than one species, the application, and analysis are difficult and complex [7]. However, the concordance index calculated by the coefficient κ was almost perfect for the samples from vectors fed with human blood between the two methods at both times, 32 and 62 days (almost perfect, 0.81-1.0) [14].
The immunochromatographic assay was useful up to 40 days of feeding with human blood in the case of using dry dejections from the vectors, which makes it a useful method for studies in peri domicile and intradomicile, where sometimes the dejections of the vectors are observed. In two states of Mexico where there is a high prevalence of Chagas disease (Ramsey et al 2015) two different species of triatomines were collected in the peridomicile and the domicile and we verified that the test is useful for more than one vector species of the disease. In conclusion, the occult blood immunochromatographic assay is useful to determine in field conditions, simply and quickly, if the vectors of Chagas’ disease are being fed by the residents of the place, so that the competent authorities take epidemiological and vector control measures.
Result
At 32 days, in all the droppings of the samples collected from groups 1, 2, 4 and 5 (total of useful samples, 108) we found the presence of human hemoglobin in the immunochromatographic assay. The droppings of groups 3, 6 and 7 yielded negative results (total of samples analyzed 81).
By heteroduplex, it was determined that in 105 of the triatomous droppings samples from groups 1,2, 4 and 5 the presence of human DNA was detected. In three of the samples, two from group 1 (human / rabbit) and one from group 2 (human / chicken) it was impossible to verify the presence of human DNA. The concordance index found between the two techniques in relation to the human food source was 32 days after ……… Table 1
In the heteroduplex analysis of the samples from groups 3 (rabbit/hen), 6 hen and 7 rabbit (total useful samples 81) the feeding source could be determined with certainty. At 62 days, in the feces of the vectors of the groups fed with human blood according to the experimental design, (groups 1,2,4 and 5) with the immunochromatographic assay, we detected the presence of human hemoglobin in 98 of the total samples useful (102 samples), while in the heteroduplex we found the presence of human DNA in 91 of the 102 useful samples, for a concordance index of ………. Table 1.
Table 1:Contingency table between heteroduplex and immunochromatographic assay at 32 days of experimentation.
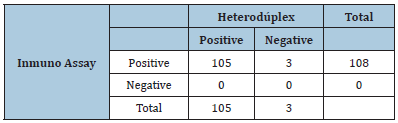
In the samples of dry excrement, dissolved in PBS, in all those belonging to insects fed with human blood, (20 in total) it was possible to determine human hemoglobin. In the experiments carried out with triatomines captured in the environment under natural conditions, we found 62 positive for human blood in the M mazzotii captured in the state of Oaxaca and 45 positive in the M pallidipennis captured in the south of the state of Puebla, Mexico.
References
- Lendrum DC, Manga L, Bagayoko M, Sommerfeld J (2015) Climate change and vector-borne diseases: What are the implications for public health research and policy? Philos Trans R Soc Lond B Biol Sci 370(1665): 20130552.
- Schofield CJ, Galvao C (2009) Classification, evolution and species group within the triatominae. Acta Trop 110(2-3): 88-100.
- Silveira A, Vinhaes M (1999) Elimination of vector borne transmission of chagas disease. Mem Inst Oswaldo Cruz 94(suppl 1): 405-411.
- Kent RJ (2009) Molecular methods for arthropod blood meal identification and applications to ecological and vector-borne disease studies. Mol Ecol Resour 9(1): 4-18.
- Chow E, Wirtz RA, Scott T W (1993) Identification of blood meals in aedes aegypti by antibody sándwich enzyme-linked immunosorbent assay. J Am Mosq Control Assoc 9(2): 196-205.
- Bosseno MF, Garcia LS, Baunaure F, Gastelum EM, Gutierrez MS, et al. (2006) Identification in triatomine vectors of feeding sources and trypanosomacruzi variants by heteroduplex assay and multiplex miniexon polymerase chain reaction. Am J Trop Med Hyg 74(2): 303-305.
- Peña VH, Fernández GJ, Gómez-Palacio AM, Mejía-Jaramillo AM, Cantillo O, et al. (2012) High resolution melting (HRM) of the cytochrome B gene: A powerful approach to identify blood-meal sources in chagas disease vectors. PLoS Negl Trop Dis 6(2): e 1530.
- Keller JI, Ballif BA, St Clair RM, Vincent JJ, Monroy MC, et al. (2017) Chagas disease vector blood meal sources identified by protein mass spectrometry. PLoS ONE 12(12): e0189647.
- Lucero DE, Ribera W, Pizarro JC, Plaza C, Gordon LW, et al. (2014) Sources of blood meals of sylvatic triatomaguasayananear zurima, Bolivia, assayed with q PCR and 12S cloning. PLoS Negl Trop Dis 8(12): e3365.
- Roellig DM, Gomez-Puerta LA, Mead DG, Pinto J, Ancca-Juarez J, et al. ( 2013) Hemi-nested PCR and RFLP methodologies for identifying blood meals of the chagas disease vector, triatoma infestans. PLoS ONE 8(9): e74713.
- Schofield CJ (1994) Triatominae: Biology & control, Eurocommunica Publications, West Sussex, United Kigdom.
- Torres-Montero J, Lopez-Monteon A, Dumontiel E, Ramos-Ligonio A (2012) House infestation dynamics and feeding sources of triatoma dimidiate in central Veracruz Mexico. Am J Trop Med Hyg 86(4): 677-682.
- Cohen JA ( 1960) A coefficient of agreement for nominal scales. Educ Psychol Meas 20(1): 37-46.
- Landis JR, Koch GG (1977) The measurement of observer agreement for cathegorical data. Biometrics 33(1): 159-174.
- Borges LV, Mattar R, Silva JMK, Silva ALW, Carrilho FJ, et al. (2018) Fecal ocult blood: A comparison of chemical and immunochemical test. Arq Gastroenterol 55(2): 128-132.
- Issa IA, Noureddine M (2017) Colorectal cancer screening: An update review of the available options. World J Gastroenterol 23(28): 5086-5096.
- Rex DK, Boland CR, Dominitz JA, Giardiello FM, Johnson DA, et al. ( 2017) Colorectal cancer screening: Recommendations for physicians and patients from the US multi-society task force on colorectal cancer. Am J Gastroenterol 112(7): 1016-1030.
- Robertson DJ, Lee JK, Boland CR, Dominitz JA, Giardiello FM, et al. (2017) Recommendations on fecal immunochemical testing to screen for colorectal neoplasia: A consensus statement by the multi society task force on colorectal cancer. Gastroenterology 152(5): 1217-1237.
- Brenner H, Tao S (2013) Superior diagnostic performance of faecal immunochemical tests for haemoglobin in a head-to-head comparison with guaiac based faecal occult blood test among 2235 participants of screening colonoscopy. Eur J Cancer 49(14): 3049-3054.
© 2021 Zumaquero Rios JL. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






