- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Evaluation of Serum Conversion Induced by Infectious Bursal Disease Vaccines Submitted to Official Quality Control in the Period 2013 to April 2014
Orsi MA1,3*, Fortunato EC1, Ashimine R, Catharino AMR1, Nascimento MLJ1, Reischak D1, Freitas TRP2 and Arns CW3
1Laboratorio Federal de Defesa Agropecuária em São Paulo (LFDA-SP), Campinas, São Paulo, Brazil
2Laboratório Federal de Defesa Agropecuária em Minas Gerais (LFDA-MG), Pedro Leopoldo, Minas Gerais, Brazil
3Laboratory of Animal Virology, Department of Genetics, Evolution, Microbiology and Immunology, Institute of Biology, University of Campinas (UNICAMP), SP, Brazil
*Corresponding author: Orsi MA, Laboratorio Federal de Defesa Agropecuária em São Paulo (LFDA-SP), Campinas, São Paulo, Brazil
Submission: November 26, 2020;Published: December 08, 2020

ISSN: 2576-9162 Volume8 Issue1
Abstract
The prophylaxis of many avian diseases is based on measures of biosecurity and active immunization using live vaccines. One of the most important tests for vaccine quality control is a serum conversion. Serum conversion is performed to check the production of certain virus, after immunization. The Avian Health Unit at LFDA-SP carries out the quality control of commercially available avian vaccines in Brazil. The aim of the study was to evaluate the results of serum conversion of live monovalent vaccines and complex Infectious Bursal Disease vaccines (IBDV) in the period of 2013 to April/2014. Twelve batches of live monovalent vaccines belonging to seven laboratories were tested and 16 batches of immune complex vaccines belonging to two laboratories. The serum conversion test was performed using two vaccine bottles. The vaccines were reconstituted in phosphate buffered saline (PBS), inoculated in SPF chicks according to the manufacturer’s recommendations and kept in BSL-3 isolators. About 21-28 days after the immunization of the chicks were bled via cardiac puncture, the sera were prepared, and analyzed by serological test (ELISA). Monovalent IBD vaccines had geometric mean titers (GMT) ranging from 648 to 10,177, combining the preparation with strong strain with a greater GMT value=10.177. The distant GMT immune complex vaccines ranging from 4,456 to 8,996. The results obtained with the monovalent and immune complex IBD vaccines, showing that all tested batches presented value required by Brazilian law and are therefore approved.
Keywords: Infectious Bursal Disease; Live vaccines; Quality Control; Serum conversion; Official Quality control
Introduction
Infectious bursal disease virus (IBDV) is the aetiological agent of an acute and highly contagious disease in young chickens. In birds of 3-6 week-old the severe disease usually is associate to the high mortality. Principal target for the virus is the lymphoid tissue with a special predilection for the bursa of Fabricius, the virus causes depletion resulting in significant depression of humoral antibody response, the birds became more susceptible to infections of other pathogens and fail to respond to vaccines OIE [1]. IBD is caused by a virus member of the Avibirnavirus genus and Birnaviridae family, viruses with bisegmented dsRNA genomes with a total of about 6 kbp forming icosahedral, non-enveloped virions Delmas B, et al. [2]. The very virulent strains causing serious disease, and to be one of the economically most important diseases that affects commercially produced chickens worldwide Müller H, et al. [3]. The prevention of IBD is based on biosecurity practices essential for the control of this disease, being related: vaccination, cleaning and disinfection of equipment and facilities, vector control, Rest between lots and traffic restriction: people/ equipment/ vehicles Cazaban C, et al. [4] The Vaccination is the principal prophylaxis measure usually are using attenuated live and killed vaccines the aviculture. Liew PS, et al. [5] Now, there is a worldwide trend of vaccination in broiler chickens in hatchery using new technology vaccines Cazaban C, et al. [4]. The vaccination in hatchery performed at the day of hatch before the day-old chickens are being placed in potentially contaminated environments. Although the ability to mount immune response, especially the adaptive immune response, is not optimal around the hatch, it is possible that the efficacy of these vaccines depends partly on innate host responses elicited in response to replicating vaccine viruses Abdul-Cader MS, et al. [6]. One of the most important tests of vaccine quality control is serum conversion, and its performed to check the production of specific antibodies to the virus after immunization. The Avian Health Unit in Laboratorio Federal de Defesa Agropecuária em São Paulo (LFDA-SP) performs the quality control of commercially available avian vaccines in Brazil. The aim of this study was to evaluate the results of serum conversion of live monovalent vaccines and complex vaccines from IBD in the period from 2013 to April / 2014.
Materials and Methods
Vaccines: 12 batches of live mononovalent IBD vaccines of the strains: Tabic, Lukert, CH80, S706, Winterfield, 228E, GBV and GM97, belonging to seven laboratories were tested and 16 batches of immune complex Gumboro disease vaccines (IBD) belonging to two laboratories.
Serum conversion test: This test was performed using two vaccine bottles. The vaccines were reconstituted in phosphate buffered saline (PBS), inoculated in SPF chicks according to the manufacturer’s recommendations and kept in BSL-3 isolators. About 21-28 days after immunization the chicks were bled via cardiac puncture. The blood was centrifuge at 1,200g/15 minutes and the sera were subjected to ELISA serological testing using the indirect ELISA Kit.
Results
Table 1: Monovalents IBDV live vaccines lots, vaccinal strains, geometric mean titers (GMT) by ELISA.

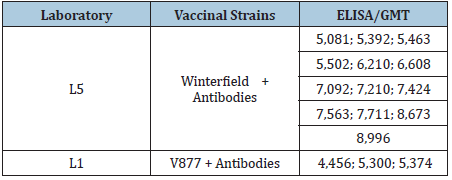
Monovalent IBDV vaccines had ELISA ranging from 648 to 10,177 (Table 1), being the best result the vaccine produced with strong strain (Winterfield) ELISA Value=10,177, following by the Tabic strains ranging from 8,253 to 8,450, 228E strain=7,793; GM97 strain=7,096; CH80 strains ranging from 5.949 to 7,025; S706 strain=6,907; Lukert strains ranging from 4,460 to 5,722, and GBV-8=648. The immune complex vaccines showed ELISA ranging from 4,456 to 8,996 (Table 2). The immune-complex vaccines of Winterfield strain the ELISA/GMT ranging 5,081 to 8,996, however the produced by V877 strain the GMT/ELISA titer ranging 4,456 to 5,374.
Table 2: Immune-complex IBD live Vaccines lots, vaccinal strains and geometric mean titers (GMT) by ELISA.
Discussion
The present paper describes the evaluation of results of GMT of titer serum conversion for measuring enzyme-linked immunosorbent assay (ELISA) induced by IBD Vaccines submitted to official quality control. The IBDV GM11 (Simbios, molecular group 11) have been detected since in 1997 in commercial broiler chickens and layer in many farms causing 2-15% of mortality severe macro and microscopic damage in different organs (cloacal bursae, spleen, thymus, kidney and liver). Scanavini NH, et al. [7], observed that IBDV antibodies were detected by ELISA test in almost all vaccinated SPF chickens before challenge while low number of commercial vaccinated and unvaccinated broilers were serologically positive (0 to 3 birds in 18). Increasing IBDV antibody titers were detected after challenge with 2050/97 strain and highest GMTs were found in broilers. It was concluded that 2050/97 strain is a highly virulent IBDV and SPF leghorn chickens immunized with BV8 intermediate vaccine strain were resistant to the challenge. Increasing susceptibility was found from experimental groups of unvaccinated broilers to vaccinated broilers and to unvaccinated SPF birds. It is discussed that passive immunity was involved in the rate of protection of challenged unvaccinated broiler and in the immune response impairment after vaccination of broilers chicks. Study realized by Rautenschlein S, et al. [8] in 2005 in commercial broiler different of the realized for us that in specific pathogen-free (SPF) with various levels of maternally derived antibodies were vaccinated with IBDV vaccines of different virulence (vaccines 1-3, intermediate; vaccine 4, intermediate plus). At an average maternal virus-neutralizing antibody (mAb) level of log210.8 (range 7.6-11.6) at day of vaccination, only the intermediate plus vaccine induced IBDV antibodies after 18 days, while the other intermediate vaccines did not. At average mAb levels of log26.7 (range 5.6-8.6) at day of vaccination, all vaccines induced circulating antibodies, although the onset of antibody production differed significantly between strains (P<0.05). While the intermediate plus vaccine induced enzyme-linked immunosorbent assay antibody levels already at 14 days postvaccination (PV), the intermediate vaccines induced significant antibody levels 28 (vaccines 1, 2) and 35 (vaccine 3) days PV. The time of IBDV antibody induction correlated with the onset of bursa lesions. The severity of lesions was comparable between vaccines 1, 3, and 4 (lesion score 4), while vaccine 2 induce only mild lesions of score 1 in 23% of the tested birds. Despite the induction of antibodies, none of the tested vaccines fully protected against challenge with vvIBDV. For the first time, it was shown that the onset of bursa lesions and recovery of IBDV-vaccinated broilers is delayed in the presence of mAb in comparison with SPF chickens but not suppressed as previously assumed. At the time of challenge, vaccinated birds may still have significant bursa lesions and may lack target cells for IBDV-challenge virus. To be able to evaluate vaccine efficacy in commercial broilers, parameters such as intrabursal IBDV-antigen load should also be considered in conjunction with bursa lesion scores. The result of antibody titer obtained in monovalent vaccines Winterfield strain for us is concordant of the find by Nishizawa M, et al. [9] the vaccine strain induced high IBD antibody titer. De Witt JJ, et al. [10] showing the results of global proficiency testing schemes (PTS) for serological tests to detect antibodies against IBDV in chicken serum, in which 125 laboratories participated from Africa, Asia, Europe, Central and South America using: virus neutralization (VN) enzyme-linked immunosorbent assays (ELISA) and agar gel precipitation (AGP). All laboratories were asked to carry out their routine diagnostic tests for the detection of IBDV antibodies as usual. This global ring trial provided a large amount of data on variation within and between laboratories and test systems used worldwide. The data showed that the variation between the quantitative test results of different laboratories (R between) using the IBDV VN test was higher (about double) compared to the variation within commercial ELISA systems. Although both tests are often referred to and used as the “gold standard”, there is an urgent need for a global implementation of recommended test procedures and/ or the inclusion of international reference sera in these studies. Camilotti E, et al. [11] in their study evaluating the pathogenicity and immunogenicity of three types of vaccines (recombinant, immune-complex and intermediate vaccines. The serum samples were submitted to ELISA to determined anti-IBD antibody titers. On day 23, chickens were submitted to the test of hypersensitivity to phytohemagglutinin to evaluate the immunosuppressive effect of vaccines on the cell-mediated immunity. The results have indicated that the immune-complex vaccine induced the most severe BF lesions, whereas the recombinant vaccine preserved BF tissue and cell integrity. The three evaluated vaccines induced humoral immunity of similar intensity [12]. The cellular reaction to phytohemagglutinin of the chickens immunized with recombinant and immune-complex vaccines was less severe compared with the unvaccinated chickens. In conclusion, these results indicate that the immune-complex vaccine was the most pathogenic and that all vaccines were effective in protecting SPF chickens against IBD. Considering the results obtained with the monovalent and immunecomplex IBD vaccines, all tested batches resented minimum values required by Brazilian legislation (9), therefore being approved.
Conclusion
These results showed the importance of the serum conversion test for the quality control of live Infectious Bursal Disease vaccines used in the country, to ensure that quality veterinary biologics are available for commercial use.
References
- OIE (2018) World organization for animal health. Infectious Bursal Disease
- Delmas B, Attoui H, Ghosh S, Malik YS, Mundtet E, et al. (2018) ICTV virus taxonomy profile: Birnaviridae. J Gen Virol 100(1): 5-6.
- Müller H, Mundt E, Eterradossi N, Islam MR (2012) Current status of vaccines against infectious bursal disease. Avian Pathol 41(2): 133-139.
- Cazaban C, Palya V & Sesti L. (2020). Doença Infecciosa da Bursa de Fabrícius (Doença de Gumboro); In: Doença das Aves. Andreatti Filho RL, Berchieri Jr A., da Silva EN, Back A et al. [Eds.], [3rd edn?] Facta, Campinas. 775-800 pp.
- Liew PS, Nurulfiza MI, Omar AR, Aini I, Bejo MH (2016) Vaccines and vaccination against infectious bursal disease of chickens: Prospects and challenges. Pertanika Journal of Scholarly Research Reviews 2(2): 23-39.
- Abdul-Cader MS, Palomino-Tapia V, Amarasinghe A, Hassan HA, Senapathi US, et al. (2017) Hatchery vaccination against poultry viral diseases: Potential mechanisms and limitations. Viral Immunol 31(3): 23-33.
- Scanavini Neto H, Ito NMK, Miyaji CI Lima E de A, Okabayashi S et.al, (2004) Infectious Bursal Disease Virus: case report and experimental studies in vaccinated and Unvaccinated SPF Chickens and Commercial Broiler Chicks. Braz. J. Poult. Sci. Brazilian Journal of Poultry Science 6(1): 41-54.
- Rautenschlein S, Kraemer Ch, Vanmarcke J, Montiel E (2005) Protective efficacy of intermediate and intermediate plus infectious bursal disease virus (IBDV) vaccines against very virulent IBDV in commercial broilers. Avian Dis 49(2): 231-237.
- Nishizawa M, Paulillo AC, Bernardino A, Alessi AC, Sayd S, et al. (2007) Evaluation of anatomopathological, serological, immunological responses and protection in broilers vaccinated with live infectious bursal disease vaccines. Arq Inst Biol 74(3): 219-226.
- De Wit JJ, van De Sande, Counotte GHM, Wellenberg GJ, (2007) Analyses of the results of different test systems in the 2005 global proficiency testing schemes for infectious bursal disease virus and Newcastle disease virus antibody detection in chicken serum. Avian Pathol 36(2): 177-183.
- Camilotti E, Moraes LB, Furian TQ, Borges KA, Moraes HLS, et al. (2016) Infectious bursal disease: Pathogenicity and immunogenicity of vaccines. Braz J Poult Sci 18(2): 303-308.
- Ministério da Agricultura. Instrução Normativa n.7 de 10 de março de 2006. Regulamento técnico para a produção, o controle e o uso de vacinas e diluentes para a Avicultura. Brasília.
© 2020 Orsi MA. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






