- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
A Recombinant Lipl21 Protein Vaccine from Five Leptospira interrogans Strains Against Pathogenic Leptospiral Infection in Vietnam
Nguyen Tuan Hung2,3, Nguyen Duc Hieu1, Dang Thị Quynh1,3, Nguyen Van Huy1,3, Nghiem Ngoc Minh1,3 and Vo Thi Bich Thuy1,3*
1Institute of Genome Research, Vietnam Academy of Science and Technology, Vietnam
2VETVACO National Veterinary Joint Stock Company, Vietnam
3Graduate University of Science and Technology, Vietnam Academy of Science and Technology, Vietnam
*Corresponding author: Vo Thi Bich Thuy, Institute of Genome Research, Vietnam Academy of Science and Technology, Vietnam
Submission: September 21, 2020;Published: October 23, 2020

ISSN: 2576-9162 Volume8 Issue1
Abstract
Leptospirosis is an emerging zoonotic disease with globally public health problem in both animals and humans. Currently, there is no effective vaccine to prevent most of the circulating serovars in Vietnam because ofthe diversity of among leptospiral serovars. In this study, a highly immunogenic epitopes from LipL21 were designed using an in-silico approach. The recombinant protein was constructed from conserved sequences of five Leptospira interrogans isolated Vietnamese strains. Immunization of mice with multi-epitope protein resulted in the antibodies production, as evidenced by MAT. Recombinant proteins can also significantly reduce Leptospire severity in animal hosts. In conclusion, rLipL21 can act as a vaccine capable of effectively and safely against the infection of five strains of Leptospira interrogans in Vietnam
Keywords: rLipL21; Recombinant protein vaccine; Leptospira interrogans; Vietnam
Abbreviations: OMPs: Outer Membrane Proteins; rLipL21: Recombinant LipL21 Protein; MAT: Microscopic Agglutination Test; LB: Luria-Bertani medium
Introduction
Leptospira Interrogans (L. interrogans) is a pathogenic bacteria that causes Leptospirosis in both animals and humans with an increasing number of cases and deaths because of the potentially fatal damage to multiple organs, including the liver, lungs, kidneys, and brain [1]. In Vietnam, Leprospirosis was first detected in humans in 1930. Currently, Vietnam is still in the high epidemiological area of Leptospirosis [2]. Epidemiological research describing this disease always has a high risk of transmitting infection to humans and animals, but for a long time it has received little attention both in terms of law and people’s awareness. The trade in animals is not strictly controlled in terms of veterinary practice, which increases the risk of disease in cattle, and human infection also increases. The major serogroups are exposed to animal in Vietnam are L. interrogans serovar Australis, Bataviae, Icterohaemorrhagiae, and Panama [3]. The vaccine for the protection of animal against L. interrogans infection have been available in Vietnam for approximately 60 years. However, there is only one inactivated vaccines type in Vietnam. Antigen is Leptospira inactivated by methiolate, including 6 strains: L. pomona, L. canicola, L. mitis, L icterohaemohagiae, L. bataviae and L. grypothyphosa. This vaccine showed limited cross-defense against strains from different serovars. Therefore, a new vaccine that can provide cross-protection for people or animals is necessary [4]. The outer membrane proteins (OMPs) are including the most major surface antigens of Leptospira [5] and can classify into three class: envelope lipoproteins, peripheral membrane proteins, and transmembrane OMPs [6]. Lipoproteins are the most common proteins among the OMPs [7,8], and LipL21 protein is an important leptospiral lipoprotein.
The LipL21 is related to the infection by acting as an adhesin and known as the host immune system targets for the antibodies production [9]. It is also conserved among pathogenic Leptospira spp. [10]. In this study, we amplified and sequenced lipL21 genes from five standard strains of Leptospira in Vietnam. The recombinant products from the LipL21 gene (rLipL21) were expressed and the mice were immunolized. Western Blot and microscopic agglutination test (MAT) were performed to detect the specific antibodies in sera to rLipL21 and the cross-immuno-agglutination against Leptospira serovars. Finally, immunoprotection of the rLipL21 was tested in mice. Taken together, our results have shown which rLipL21 could be used as potential candidate of a vaccine against Leptospirosis in livestock. gene (rLipL21) were expressed and the mice were immunolized. Western Blot and microscopic agglutination test (MAT) were performed to detect the specific antibodies in sera to rLipL21 and the cross-immuno-agglutination against Leptospira serovars. Finally, immunoprotection of the rLipL21 was tested in mice. Taken together, our results have shown which rLipL21 could be used as potential candidate of a vaccine against Leptospirosis in livestock.
Materials and Methods
Bacterial strains and culture conditions
Five Leptospira interrogans serovar Pomona, Canicola, Icterohaemohagiae, Bataviae and Grippotyphosa isolates was stored in Vietnam National Center for Veterinary drugs and Bio- Products Controls No.1. All the leptospiral strains were cultured in Korthof medium at 28 oC. Escherichia coli strains DH5 alpha and BL21 were grown at 37 oC in Luria-Bertani (LB) medium. 100μg/ mL ampicillin was added in LB agar medium for screening vector transfected cells.
Sequencing of lipL genes of five Leptospiral strains
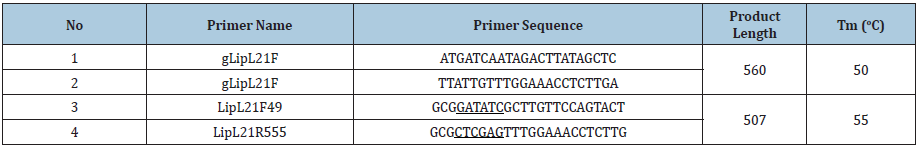
Leptospiral DNA was extracted using GeneJET Genomic DNA Purification Kit (Thermo Fisher Scientific, USA). Primers for amplification of lipL21 gene is shown in Table 1. The 50μl total volume per PCR reaction was follow the manual of 2X PCR Master Mix (Thermo Fisher Scientific, USA). The reaction mixture was initiated by denaturate at 94 oC for 5min, followed by 30 cycles of amplification, and then finish at 72 oC for 10min. The PCR products were purified using the PCR Products Purification Kit (Biocolor, UK) and then sequenced using ABI3500 system (Applied Biosystems, CA, USA). The alignment of sequncing results were performed by the CLUSTALW algorithm of MEGA6 software.
Table 1: LipL32 primer list.
In-silico prediction and selection of vaccine epitopes
The sequences of LipL21 were collected from conserved sequence of five Vietnam isolates. The protein structure was predicted using SWISS-MODEL. The antigenic epitopes were predicted using BepiPred Prediction Software in IEDB Analysis Resource server (http://tools.iedb.org/).
Physicochemical properties
The protein was predicted and further submit to ProtParam tool (http://web.expasy.org/ tools/protparam/protpar-ref.html) for the prediction of the amino acid composition, molecular weight, instability index, aliphatic index and grand average of hydropathicity of the protein sequences.
Cloning and expression of rLipL21
Restriction enzyme sites were inserted in the amplified primer pair. The amplified product was then cloned into pJET1.2 cloning vector by CloneJET PCR Cloning Kit (Thermo Fisher Scientific, USA) and confirmed by sequencing with an ABI3500 system (Applied Biosystems, CA, USA). After sequence confirmation, all the genes were subcloned into pET32a. One day before expression, cells were cultured overnight in LB added ampicillin at 37 oC. In expression day, overnight culture was diluted to 1:100 and refresh culture until OD=0.6 by incubated for 90min at 37 oC and 200rpm. After that, the culture medium was added 1 mM IPTG (final concentration) incubated for 4h (at 37 oC and 200rpm). Cells were collected by centrifugation at 12,000×g for 10min at 4 oC and the pellets was stored at -20 oC. The E. coli cell pellets were resuspended in 10 mL of PBS buffer and sonicated on ice five times for 30s and 30s break. The whole cell lysate was centrifuged at 12,000×g for 10min at 4 oC and the supernatant was purified by Ni-NTA Superflow Columns (Qiagen, USA). Purified protein was then eluted using elution buffer.
Production of antiserum against rLipL21
A group of eight 6-week-old mice were purchased from the laboratory animal breeding of VETVACO Join Stock Company, Vietnam. The mice were healthy and uninfected with Leptospira that immunized intraperitoneally with 100mg of rLipL21, mixed with Freund’s adjuvant (Sigma Aldrich Co., St. Louis, MO) in four following doses (on days 0; 14; 21; and 28). Blood samples were collected from the retro-orbital venous plexus one week after each immunization and stored at -20 oC for the next immunology analysis.
To determine the effectiveness of vaccine-induced protection, the immunized mice were challenged with a dose of 103 leptospires, equivalent to five times the 50% lethal dose (LD50) of the all Leptospira interrogans serovar Pomona, Canicola, Ictero haemohagiae, Bataviae and Grypothyphosa.
Microscopic agglutination test MAT analysis (MAT)
The sera from immunized mice were diluted into 1:50 in PBS and mixed with the same volume of Leptospira cultures from five reference strains of Leptospira in Vietnam. After incubated at 37 oC for 1h, agglutination was observed by dark-field microscopy. The agglutination rate more than 50% was determined which sera was positive reactivity against five pathogenic serovars.
SDS-PAGE and Western blot for the evaluation of antibody response
The purified target protein was run on 10% SDS-polyacrylamide gel and transferred to a nitrocellulose membrane. The membranes were then blocked with PBS containing 0.05% Tween-20 and 5% dry milk. The bound proteins were detected using mouse antiserum (diluted 1:12,000), and incubated for 1h at 37 oC. After washing, horseradish peroxidase-conjugated anti-mouse immunoglobulin G (diluted 1:3000; Santa Cruz Biotech, CA, USA) was added as a secondary antibody and incubated at 37 oC for 2h and washed three times with PBST. The membranes were visualized using o-phenylenediamine dihydrochloride (Sigma-Aldrich, St. Louis, MO, USA) and hydrogen peroxide reagent.
Histological analysis
Tissues from mice were preserved in 1% formaldehyde solution for one month, and then embedded in paraffin. The fixed kidney and liver tissues were cut to 5μm sections with the aid of a microtome. Tissue sections were stained with hematoxylin and eosin (H&E) using following the standard histological protocol, and the tissue image was recorded by light microscopy camera.
Statistical analysis
The survival rates in challenge experiments were analyzed by using GraphPad Prism 5 software.
Result
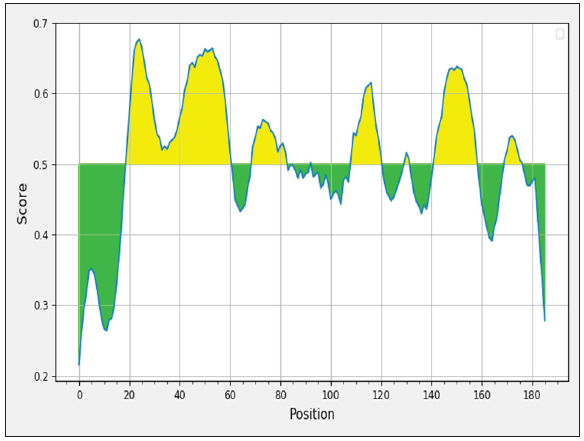
Prediction and selection of vaccine epitopes
The sequencing results showed the similarity between five sequences was 99% with six polymorphism position at nucleotide 99; 171; 276; 279; 324; and 450. However, all variances were nonsense and the amino acid sequences were similar between five Leptospira strains. In-silico bioinformatics tools were used to select the multi-epitope region. Antigenic epitopes (Table 2) from LipL21 glycoproteins were predicted, and the amino acid sequence from 17 to 185 position (equivalent to nucleotide sequence from 49 to 555 position) was selected (Figure 1).
Table 2: LipL21 Predicted epitopes.
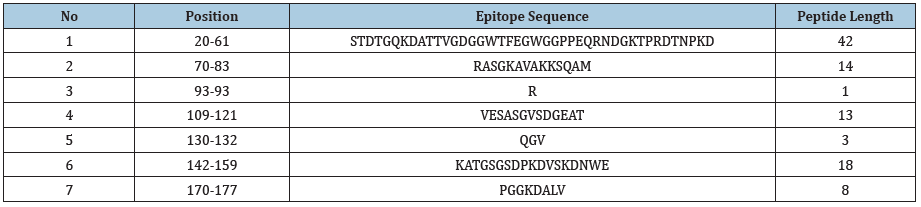
Figure 1: Potential B cell epitopes for LipL21 gene.
Physicochemical properties
Using ProtParam tool to predict the physical and chemical properties of the protein rLipL21. The instability index was smaller than 40 and the aliphatic index was 50.18 showed the protein is stable. However, the minus value of grand average of hydropathy showed the solubility of this protein was low. Parameters were shown in Table 3.
Table 3: Physicochemical properties of amino acid sequences based on the Prot param.

Expression, purification of the chimeric rLipL21 protein
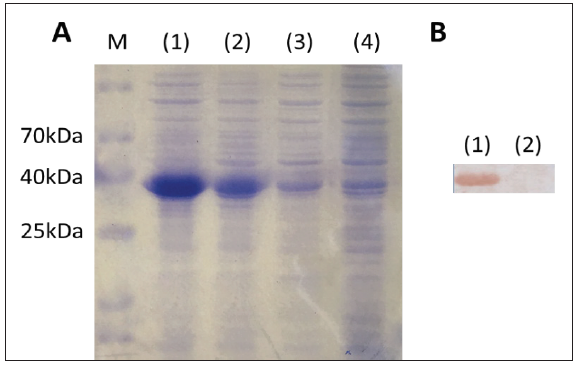
The rLipL21 gene fragment, after isolated by treating the pJET1.2-rLipL21 vector with restriction enzyme restriction enzyme (Figure 2) was successfully ligated into the pET32a expression plasmid at position downstream of the Signal Peptide and upstream of His tag. The protein was successfully expressed in E. coli BL21 and purified. SDS-PAGE results showed that the fulllength 36.751kD protein (the combinate of rLipL21, Signal Peptide and His tag) was induced by IPTG and presented in both the pellet and supernatant fraction of lysed E. coli BL21 cells. Besides, SDSPAGE analysis of the purified protein with mice antiserum showed a single band (Figure 3).
Figure 2: pJET1.2-LipL21 and pET32 vector (A): pJET1.2-LipL21 vector; (B): pET32 vector Lane (1) non restriction enzyme treatment; lane restriction enzyme treatment.

Figure 3: Characterization of the expressed of LipL21 protein. (A). SDS-PAGE analysis of the expression of the LipL21 protein. Lane (1) pellet and lane (2) supernatant fractions of lysates from induced E. coli BL21 cells; lane (3)(4) non induced BL21 cells. (B). Western Blot analysis. Lane (1) purified rLipL21; lane (2) PBS.
Microscopic agglutination test MAT
Agglutinating antibody titres was measured based on their reactivity against five pathogenic serovars. The antibody response was measured by calculating the mean titre for both immunized and control groups. The observed results showed positive reactivity against antigens from serovars L. pomona, L. canicola, L. mitis, L. icterohaemohagiae, L. bataviae and L. grypothyphosa of rLipL21 immunized group at the 1:50 dilutions compare to PBS groups.
Challenge experiments
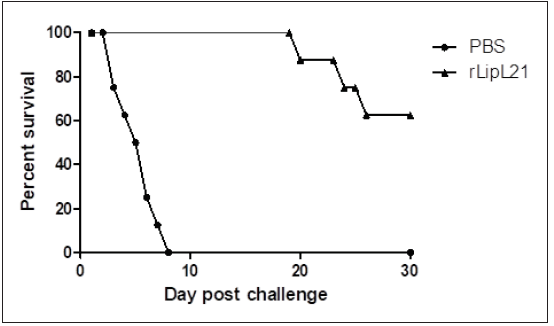
The protective efficacy of rLipL21 in mice against five Leptosipra strains was determined in a challenge experiment. The survival analysis showed 62.5% and 0% survival among mouse groups immunized with rLipL21 and PBS respectively (Figure 4).
Figure 4: The survival of challenge mice.
Histopathological analysis
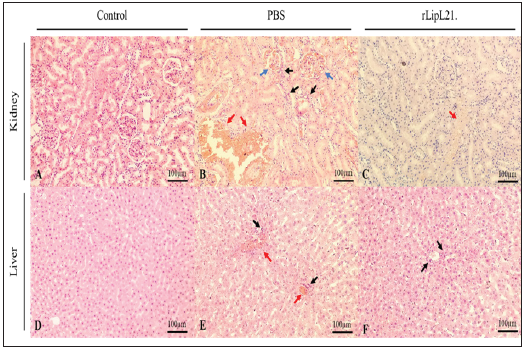
Based on the histopathological analysis, the intensity of the lesions for the vaccinated groups was less severe compared to the challenged control group. The challenged PBS-immunized mice showed severe Leptospirosis symptoms including histopathological lesions in the kidney and liver, inflammatory infiltrates like lymphocytes, plasma cells and macrophages, hemorrhages, and vascular engorgement. In contrast, liver, kidney and lung tissues of rLipL21-immunized animals showed only mild symptoms as vascular engorgement in kidney or inflammatory infiltrates in liver (Figure 5).
Figure 5: Photomicrographs of kidney and liver sections. Black arrow: inflammatory infiltrates; Blue arrow: hemorrhages; Red arrow: vascular engorgement.
Discussion
Leptospirosis is known as an emerging zoonotic disease because it can spread rapidly in rural areas and even urban areas of industrialized and developing countries [11]. The development a new tools for leptospirosis treatment, especially an effective vaccine, would be considered the best solution against Leptospira. However, previous researches showed the weak cross-immunoprotection of antigens from traditional vaccine to different serogroups and serovars of pathogenic Leptospira species [12]. In addition, both inactivated and attenuated vaccines are associated with potential risks, such as aches and anaphylaxis, and the serovarspecific immunity is only short-term [4]. Therefore, identification of universal protein antigens for all pathogenic Leptospira species circulating in Vietnam and development of a more effective vaccine would replace the traditional vaccines to controlling the disease. Antibodies play the important role in the mechanism of phagocytosis and destruction again pathogenic Leptospira [13]. Nowadays, by using in-silico bioinformatics, we can predict and engineer synthetic proteins to induce the immune response and the produced antibody can be specific to various pathogenic serovars which has the highly complex antigenic diversity and make more long-term of vaccine efficiency. The construction of new recombinant vaccine may potentially increase efficacy than the natural protein sequences [14]. Beside, synthesized outer membrane protein could produce a significant induce on the immuno-reactivity against diverse leptospiral serovars [15]. In our study, after predicting epitope and selecting the suitable region of LipL21 gene for protein expression, SDS PAGE and Western blotting, which are very common methods for studies to identify the efficiency of epitopes to B-cell, were used. Our results revealed that the immunogenicity of rLipL21 strongly express in mice and specific antibodies were produced. The MAT, a “golden standard” test for Leptospira serodiagnosis of and serological classification, was used to confirm that antibodies can link with epitopes of all five leptospiral serovars [16]. The titer at 1:50 was selected as standard dilution for MAT test and results showed the agglutination of rLipL21 antisera with all standard reference strains of Leptospira in Vietnam. Therefore, we expect that antibodies raised after rLipL21 vaccination are able to provide cross-protection against a wide variety of Leptospira serovars, although this still requires further confirmation using animal experiments.
Using rLipL21 protein as vaccine, 62.5% of the mice survived after the challenge experiment. The similar of our results with previous researches [17] showed that mice immunized with our rLipL21 vaccine recognized the Leptospira and were cross-protected against leptospirosis, demonstrating the potential of our protein as a cross-protective antigen [18]. Moreover, LipL21 amino acid sequence was conserved among five strains, with 100% pairwise identity. The protection of the immunization with rLipl21 and the histopathological lesions from Leptospira colonization on kidney and liver of challenged mice were evaluated. The histopathological images showed more severe lesions the control group than the vaccinated. There were several factors could cause the tissue lesions including challenge method, dose and virulence of tested strains. However, previous evidence supported that vaccinated mice can develop protective immunity. Therefore, the activate of the immune response including inflammasome activation [19,20], cytokines production [21], and autoimmune reactions [22], can impart resistance to colonization and reduce the tissue damage[23]. To the best of our knowledge, this is the first research of rLipL21 production as a vaccine candidate against Leptospirosis in Vietnam. However, further studies are needed to evaluate the effectiveness of this recombinant protein before it can be produced as a complete vaccine used in livestock production.
Conclusion
This study showed that the potential of rLipL21 as a multiepitope antigen against five common Leptospira strains in Vietnam. Immunized mice showed a strong antigen-specific immune response, a reduction in tissue damage and colonization and an increasing survival rate in challenge experiments. Thus, the rLipL21 may be recommended as promising vaccine candidate against Leptospirosis.
Acknowledgement
This research was funded by the VETVACO Join Stock Company, Vietnam.
References
- Dutta TK, Christopher M (2005) Leptospirosis-An overview. J Assoc Physicians India 53: 545-551.
- Loan HK, Van Cuong N, Takhampunya R, Kiet BT, Campbell J, et al. (2015) How important are rats as vectors of leptospirosis in the Mekong Delta of Vietnam? Vector Borne Zoonotic Dis 15(1): 56-64.
- Lee HS, Khong NV, Xuan HN, Nghia VB, Nguyen VH, et al. (2017) Sero-prevalence of specific leptospira serovars in fattening pigs from 5 provinces in Vietnam. BMC Vet Res 13(1): 125.
- Langston CE, Heuter KJ (2003) Leptospirosis: A re-emerging zoonotic disease. Vet Clin North Am Small Anim Pract 33(4): 791-807.
- Cullen PA, Haake DA, Adler BJF (2004) Outer membrane proteins of pathogenic spirochetes. FEMS Microbiol Rev 28(3): 291-318.
- Gebriel A, Subramaniam G, Sekaran SJTB (2006) The detection and characterization of pathogenic leptospira and the use of OMPs as potential antigens and immunogens. Trop Biomed 23(2): 194-207.
- Cullen PA, Cordwell SJ, Bulach DM, Haake DA, Adler BJI, et al. (2002) Global analysis of outer membrane proteins from Leptospira interrogans serovar Lai. Infect Immun 70(5): 2311-2318.
- Cullen PA, Xu X, Matsunaga J, Sanchez Y, Ko AI, et al. (2005) Surfaceome of Leptospira spp. Infect Immun 73(8): 4853-4863.
- Lin Xa, Zhao J, Qian J, Mao Y, Pan J, Li L, et al. (2010) Identification of immunodominant B-and T-cell combined epitopes in outer membrane lipoproteins LipL32 and LipL21 of Leptospira interrogans. Clin Vaccine Immunol 17(5): 778-783.
- Seenichamy A, Bahaman AR, Mutalib AR, Bejo KS (2014) Production and characterization of a polyclonal antibody of anti-rLipL21-IgG against Leptospira for early detection of acute leptospirosis. BioMed Res Int 2014.
- Cruz LS, Vargas R, Lopes AAJE (2009) Leptospirosis: A worldwide resurgent zoonosis and important cause of acute renal failure and death in developing nations. Ethn Dis 19(1 Suppl 1): S137-S141.
- Samina I, Brenner J, Moalem U, Berenstein M, Cohen A, et al. (1997) Enhanced antibody response in cattle against Leptospira hardjo by intradermal vaccination. Vaccine 15(12-13): 1434-1436.
- Bharti AR, Nally JE, Ricaldi JN, Matthias MA, Diaz MM, et al. (2003) Leptospirosis: A zoonotic disease of global importance. Lancet Infect Dis 3(12): 757-771.
- Yu K, Liu C, Kim BG, Lee D-YJ (2015) Synthetic fusion protein design and applications. Biotechnol Adv 33(1): 155-164.
- Rollauer SE, Sooreshjani MA, Noinaj N, Buchanan SK (2015) Outer membrane protein biogenesis in gram-negative bacteria. Philos Trans R Soc B 370(1679): 20150023.
- Xiao G, Luo D, Kong L, Chen X, Sun D, et al. (2016) Chimeric epitope vaccine against Leptospira interrogans infection and induced specific immunity in guinea pigs. BMC Microbiol 16(1): 1-9.
- Kumari A, Premlatha MM, Raja V, Mercy CSA, Sumathi K, et al. (2018) Protective immunity of recombinant LipL21 and I-LipL21 against Leptospira interrogans serovar Autumnalis N2 infection. J Infect Dev Ctries 12(1): 22-30.
- Srikram A, Zhang K, Bartpho T, Lo M, Hoke DE, et al. (2011) Cross-protective immunity against leptospirosis elicited by a live, attenuated lipopolysaccharide mutant. J Infect Dis 203(6): 870-879.
- Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, et al. (2010) The NLRP3 inflammasome promotes renal inflammation and contributes to CKD. J Am Soc Nephrol 21(10): 1732-1744.
- Xiang M, Shi X, Li Y, Xu J, Yin L, et al. (2011) Hemorrhagic shock activation of NLRP3 inflammasome in lung endothelial cells. J Immunol 187(9): 4809-4817.
- Matsui M, Rouleau V, Ostells BL, Goarant C (2011) Gene expression profiles of immune mediators and histopathological findings in animal models of leptospirosis: comparison between susceptible hamsters and resistant mice. Infect Immun 79(11): 4480-4492.
- Verma A, Stevenson B (2012) Leptospiral uveitis-there is more to it than meets the eye! Zoonoses Public Health 59 (Suppl 2): 132-141.
- Zuerner RL (2015) Host response to leptospira infection. Curr Top Microbiol Immunol 387: 223-250.
© 2020 Vo Thi Bich Thuy. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






