- Submissions

Full Text
Approaches in Poultry, Dairy & Veterinary Sciences
Effect of Organic Selenium Dietary Supplementation on Growth Performance, Carcass Retention and Meat Lipid Peroxidation in Broilers
Chalghoumi R1#, Beckers Y2, Schoeling O2, Seal BS3, and Théwis A2
1Laboratory of Improvement and Integrated Development of Animal Productivity and Food Resources, School of Higher Education in Agriculture of Mateur, University of Carthage, Bizerte, Tunisia 2Precision Livestock and Nutrition Unit, Gembloux Agro-Bio Tech, University of Liege, Belgium 3Oregon State University Cascades, USA
*Corresponding author: R Chalghoumi, Laboratory of Improvement and Integrated Development of Animal Productivity and Food Resources, School of Higher Education in Agriculture of Mateur, University of Carthage, Bizerte, Tunisia
Submission: September 4, 2020;Published: October 15, 2020

ISSN: 2576-9162 Volume7 Issue5
Abstract
This study was conducted to determine the effect of dietary Selenium (Se) supplementation on growth performance, carcass Se retention and meat lipid peroxidation in commercial broiler chickens.A total of 216 1-day-old male Cobb broiler chicks were randomly divided into 6 groups of 36 chicks each (6 chicks × 6 replications) and assigned to one of the following treatments: (1) a conventional diet as a control (C), (2) C diet supplemented with increasing concentrations (1,2,3,4 or 6mg/kg diet) of organic Se (D1, D2, D3, D4 and D5).Se-supplemented diets were only fed during the grower-finisher phase (d15-d42). Body weight gain, feed intake and feed conversion ratio were not affected by Se supplementation either during the grower-finisher phase or during the overall rearing period. Se concentration among 42-d-old chickens’ carcasses increased significantly and linearly from 0.11 (C) to 1.44 (D5)mg/kg live body weight. The amounts of ingested, retained and excreted Se also increased significantly and linearly with dietary Se supplementation level.Se retention efficiency was low ranging from 10.5% (D4) to 20.6% (D2). No difference was detected between dietary treatments with respect to lipid peroxidation after a 7-day storage period either in the pectoralis or thigh muscle. These results indicate that dietary supplementation with organic Se did not influence growth performance nor lipid peroxidation status.However, it increased the Se content of broiler chickens’ carcasses. Thus, this approach could be used to produce Se-enriched meat in order to improve the Se status of consumers
Keywords: Broiler chicken; Carcass; Feed supplementation; Growth performance; Lipid peroxidation; Organic selenium
Introduction
The presence of trace elements in the human body is now proving to be of paramount importance for the body. These trace elements ensure to the body the maintaining of its structural integrity and optimal biochemical function.Selenium (Se), for example, reportedly has preventive and therapeutic potential against heart disease, cancer and many other degenerative pathologies with an oxidative component [1]. In several countries worldwide, natural intake of Se are modest enough (40-60μg/day) and sufficient to avoid the risk of true deficiencies. However, these intake levels do not prevent the development of certain pathologies, highly widespread such as cancer and cardiovascular diseases. According to the Ministry of Public Health [2],cardiovascular diseases are the leading cause of death in Tunisia, responsible for 1 death on 3 per day. Thus, an increased intake of Se would be desirable.
Se supplementation is a controversial subject. Although in the past decade considerable knowledge has been accumulated about the role of Se in human health, uncertainty still reigns. This uncertainty is linked to the gap between need and excess, which appears to be relatively narrower than for other trace elements [3,4].In addition, the toxicity threshold of this nonmetal is difficult to set because of a number of complex factors. The chemical form in which the element is ingested determines its effects on health. In this respect, organic forms seem to be less toxic than inorganic ones[5]. The quantity and nature of proteins in the diet, the presence of vitamin E and heavy metals also affect the toxicity of Se. However, the biochemical processes of these interactions are largely unknown.
The enrichment of agricultural products makes it possible to provide the consumer with organic Se as naturally occurring in food and is perfectly adapted for digestion and metabolism. Se is not essential for the plants[6] but is an essential element for animal feed. Indeed, Se deficiency is the cause of many diseases and abnormalities, such as exudative diathesis and pancreatic fibrosis in broiler chickens[7,8]. These pathologies have a negative impact on animals’ performance and the quality of their products. The national research council (NRC,1994) [9]recommended 0.15mg/kg as Se supplementation level for broilers. Although this level is reportedly not adequate for modern industrial poultry production[7].
The present study was designed to define the upper safe limit dose of organic Se to be incorporated into the broiler chicken diet in order to achieve the optimal Se retention into the carcass. The overall goal was to ultimately produce Se-enriched meat without compromising growth performance. A second concern was to evaluate the impact of this supplementation on meat lipid oxidative status.
Materials and Methods
Birds and experimental design
Two hundred and sixteen 1-d-old male Cobb broiler chicks from a local commercial hatchery were used in this study. Upon their arrival, chicks were randomly divided into 6 groups in a completely randomized design (6 treatments × 6 replications, each replication included 6 chicks). The 6 groups were allocated as follows: (1) a control group fed a conventional feed for broiler chicken (C) containing 0.15mg Se/kg (Table1,2)five experimental groups (D1,D2,D3,D4 and D5) fed the control feed supplemented with increasing amounts of organic Se. Organic Se is incorporated into the feed as Se-enriched yeast (Sel-Plex, Alltech, Inc) (Table 2).
Table 1: Ingredients and chemical composition of the control diet (C).
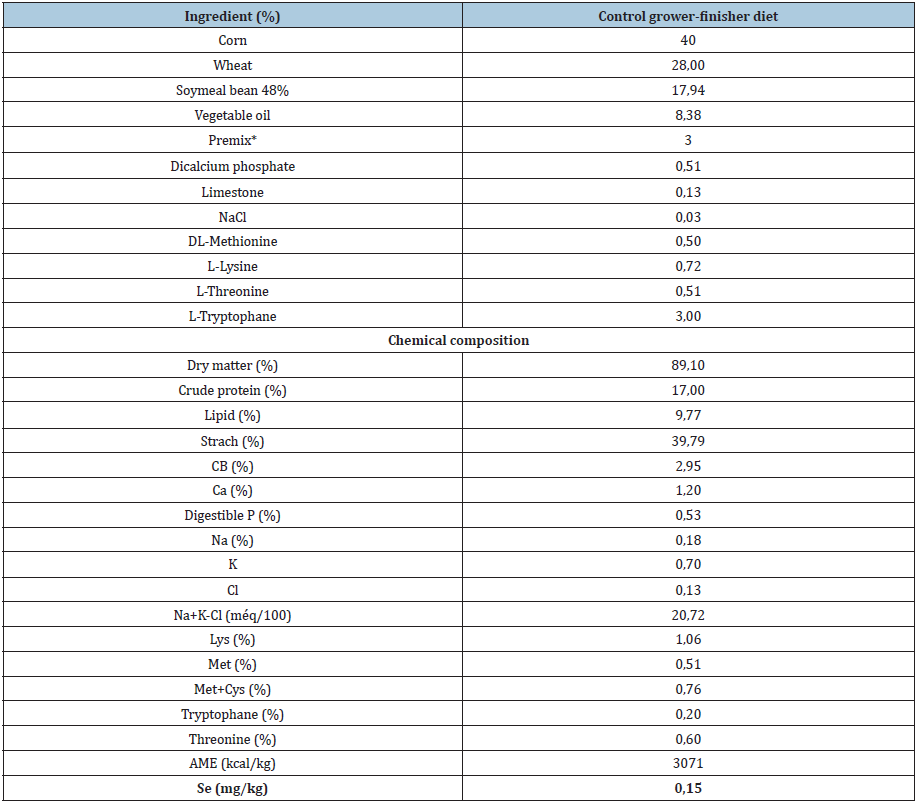
*Premix F-0. 1-3, Comptoir de Belgique, Belgium, provides: Ca 24%, P 10%, Na 1%, Mg 0,1%, I 16 mg/kg, Co 16 mg/kg, Fe 1250 mg/kg, Mn 2660mg/kg, Zn 2333 mg/kg, 3 mg/kg, Vit A 333000 UI/kg , Vit D3 66600ui/kg Vit E 500 mg/kg , Vit B1 33mg/kg Vit B2 200mg/kg, Vit B3 266mg/kg, Vit B6 33mg/kg Vit B12 0,67mg/kg, Vit K2 33 mg/kg, acide folique 17 mg/kg, choline 17000 mg/kg, méthionine 4,5%.
Table 2: Se supplementation level of the experimental diets.

1Sel-Plex, Alltech, Inc. Selenium content =2000 mg. Kg-1 of yeast.
The trial was performed in two phases: a starter phase (d1- d11) and a grower-finisher phase (d12-d42). During the starter phase (d1-d11), all the chicks received the same starter feed containing 0.15mg Se/kg. On days 12 and 13, a transition period was applied to allow animals to adapt to their new feeds. From day 14 to day 42, each chicken group received its corresponding “growth-finisher” feed. Birds were given ad libitum access to feed and water throughout the whole rearing period.
The chicks were reared in an environmentally controlled room in 1.1×1.2-m pens. They were maintained on a 24-h light schedule (23-h/1-h light/dark cycle). The ambient temperature was initially raised to 34°C, and gradually decreased to 20°C by the end of the trial. Ventilation was automatically regulated.All procedures related to animals’ care, handling, and sampling were conducted under the approval of the Official Animal Care and Use Committee of the Gembloux Agro-Bio Tech, University of Liege (Belgium) before the initiation of research and followed the Belgian official guidelines. Experimental Measures
Growth performance
Birds’ live body weight (LBW) and feed refusal were determined weekly on d14, d21, d28, d35 and d42 to calculate body weight gain (BWG), feed intake (FI) and feed conversion ratio (FCR) on individual basis. Mortality was recorded daily and birds that died were noted along with recording their body weight that was used to adjust the FCR accordingly.
Se apparent balance
The amount of Se ingested over the entire rearing period (d1- d42) was calculated as the sum of the amounts of Se ingested during the starter phase (d1-d11) and growing-finishing phase (d12-d42). For each of the 6 birds’ groups, Se ingested during each period was calculated by subtracting Se content of feed refusal from Se content of the distributed feed. Se retained was calculated by subtracting the amount of Se contained in the carcass of 1-day-old chicks from that of Se in the carcass of 42-day-old chickens. The amount of Se in the carcass is calculated by multiplying the LBW of the birds by their Se content, expressed in mg per kg of LBW. The Se excreted was calculated by subtracting the amount of Se retained in the carcass from the amount of Se ingested by birds.
Before starting the experiment, six 1-d old chicks were randomly selected from surplus animals and euthanized by CO2 asphyxiation so that the carcasses were recovered intact in order to determine the carcass Se content on d1. At the end of the feeding trial (d42), 6 birds randomly selected per treatment (1 bird randomly selected per pen) were also euthanized in the same manner in order to determine the carcass Se content on d42.After euthanasia, carcasses were placed directly into specific bags for autoclaving, weighed and autoclaved for 5hours at 121°C. Thereafter, carcasses were weighed again and milled using a homogenizer (Homogenizer 1094, Tecator). The homogenized material was recovered in a previously tared container, weighed, stored at -20°C then freeze-dried for analyses. The dry sample obtained was roughly reduced using a homogenizer (Homogenizer 1094, Tecator). Part of the ground material (±60g) was recovered, degreased (cold rinsing with diethyl ether) and then ground to 0.5mm in a fast rotor mill (Spray 14, Fritsch). Finally, the powder obtained was stored in a sealed jar for the subsequent determination of Se content of carcasses.
Se content of feed, feed refusals, and whole carcass (on d1 and d42) was measured by flame atomic absorption spectrometric method[10]. The assay was carried out in three steps according to the following procedure. At first, the sample was wet-mineralized. 0.5g of sample is weighed in a 100ml flask. 10ml of acids mix [1/3 nitric acid (HNO3) 65%, 2/3 hydrochloric acid (HCl) 37%] were added. The sample was left to macerate overnight at room temperature. Finally, the mixture was boiled for 2h 30min. After partial evaporation of the acid mix, the mineralized material was filtered through a filter paper (S & S 595 1/2) and transferred into a 50ml volumetric flask. Then, the volume of mineralizer was raised to 50ml by adding distilled water. From each vial, a sample was taken into a 14ml polypropylene tube (Falcon). The tubes were then stored in a cold room at 4°C. From each 14ml sample, 1ml was taken into a test tube to which 0.5 or 1ml of hydrochloric acid (37% HCl) was added, depending on the Se content in the sample. The tube was placed in a sand bath and heated at 80°C for 20-45min. After cooling, the volume of the test tube was raised to 5ml. Finally, the absorbance was measured at 340nm by means of a flame atomic absorption spectrometer (PerkinEmmer Analyst 800) coupled with an FIMS system (Flow Injection Mercury System). Controls and standards were provided and treated in the same way as samples. The calculation was carried out using a computer software taking into account the sample weight and the dilution factor.
Meat lipid peroxidation
On d42, 18 chickens (3 chickens per treatment) were randomly selected, slaughtered and dissected. The pectoral and thigh muscles were carefully removed, rinsed in an ice-cold solution of potassium chloride (KCl) 0.15% to remove residual blood, and then wiped clean. The samples were then packed in small racks, identical to those used for the sale of pre-packaged meat in supermarkets. Thereafter, samples were placed for 7 days under conditions close to those found in the “refrigerator-shelves” in supermarkets, that is in a cold room (0 to 4°C) lit by neon lights.
At the end of the storage period (7 days), the degree of lipid peroxidation of the pectoral and tight muscles was estimated by measuring malondialdehyde (MDA) production through thiobarbituric acid (TBA) index analysis[11]. The analysis involved muscle lipids only and the connective and fatty tissues were removed before processing. The sample was quickly minced in a mill (Moulinex, France). Ten grams were then weighed (Precisa XT4200C); 1ml of butyl-hydroxytoluene (BHT) and 40ml of distilled water were added to the sample. The mixture was homogenized for 30 seconds (Silverson SL2T) and transferred into a “Büchi” tube. An antifoam pellet and 3ml HCl were added to the mixture. The mixture was then distilled on distillation units (Büchi, Flawil, Switzerland). The MDA was moved by steam and entrained in a graduated flask (100ml). In a test tube, 5ml of the MDA sample was taken and 5ml of TBA (0.02M) were added; the tube is closed and placed in a water bath (100°C) for 35min. Finally, the absorbance was measured at 532nm using a spectrophotometer (Pye Unicam PU 8600 UV/VIS spectrophotometer, Philips). Each distillation serie, included two controls and standards (5 to 25nmol/5ml) to evaluate the importance of the possible loss of MAD and to plot the concentration of MAD standards as a function of absorbance measurement, respectively. The amount of MDA in the samples was expressed in mg/kg of muscle.
Statistical analysis
The collected data were subjected to analysis of the variance using a completely randomized model and processed by the GLM procedure of the SAS 6.12 software [12] to evaluate the effect of Se dietary supplementation level on experimental variables measured. Linear correlations between the parameters considered were calculated by the Pearson correlation test. Results were considered significant if P<0.5.
Results and Discussion
Mortality
The mortality rates recorded during the whole trial ranged from 2.78% to 8.33% and this mortality was not correlated to the Se dietary supplementation.The highest mortality rate (8.33%) was observed in chickens fed the un-supplemented diet C and D3 (C+3ppm). On the other hand, the lowest rate (2.78%) was recorded in chickens fed D2 (C+2ppm) and D5 (C+6ppm). This seems very interesting regarding to the tolerance of broilers to high intake of Se relative to the D5 diet. Indeed, the Se content of this diet was significantly higher than the maximum content (i.e. 0.15mg Se/kg DM) allowed by the legislation for broiler chickens feed [13] and the maximum supplementation level (5ppm) recommended in the literature as the limit between a toxic and non-toxic Se intake in poultry and other monogastric animals [14]. Therefore, it seems that incorporation of Se at supplementation level up to 5ppm in a basal broiler diet has no lethal effect. This observation was similar to that reported by Echevarria MG, et al.[15,16] who did not observe any toxic effect, expressed in term of mortality, when they added Se to the feed at an incorporation level of 9ppm. In that study, the investigators used inorganic Se and animals were younger (1 and 3 weeks of age). In another study conducted by TodorovicM, et al. [17] who fed 1-day-old chicks for 6 weeks with a basal diet supplemented with Se (sodium selenite) at supplementation level of 0,2,5,10,15,20 and 30ppm, only the three highest supplementation levels caused high mortality rates of 26,60 and 80%, respectively. Deniz G, et al. [18] reported that mortality was not significantly altered by Se supplementation using organic or inorganic Se forms.
Mortality observed during this trial may be related to other factors. Leg problems were frequently observed and seem to be the main cause of mortality. Indeed, animals with leg weakness have difficulty in accessing feed, become prostrate and die. Since birds suffering from this disorder do not systematically belong to groups receiving the most Se enriched diets, we cannot conclude that this disorder is related to the dietary Se supplementation. However, it is important to note that during the dissection of some birds that received the diets having the highest levels of Se, i.e. D3,D4 and D5, reddish spots were observed, particularly on the pectoral muscles and the thigh. Whitish spots were also observed on the liver. This could be related to Se. Indeed, some authors report that the administration of relatively high doses of Se to broiler chick is often associated with histological changes [19,20].
Growth performance
Data relative to the effect of dietary Se supplementation on growth performance recorded during the experimentation are reported in Table 3. No significant differences were determined between dietary groups in feed intake, BWG and FCR (P>0.05) either during the grower-finisher phase or during the overall rearing period. Feed intake over the entire trial period averaged 83.55g/d with a minimum of 81.77g/d observed for chickens fed D5 and a maximum of 84.70g/d recorded for those fed control diet (C). The maximum value of BWG was observed in chickens receiving the D3 diet (61.9g per chicken per day). The minimum value was observed in chickens fed the D5 diet (56.79g per chicken per day). The highest FCR was obtained in birds that received D1 (1.45), while the lowest value was recorded for birds fed D3 diet (1.36).Table 3: Effect of Se dietary supplementation on growth performance of broiler chickens during overall experimental period (d1-d42).
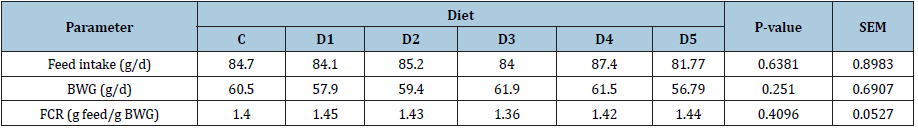
It can be concluded that dietary Se supplementation at levels exceeding the NRC [9] (1994) requirement (0,15ppm, control diet) and up to 4.15ppm (D4) had no negative impact on feed intake, BWG and FCR. This finding is partially consistent with that of Echeverria MG, et al. [16] who observed a significant decrease in feed intake and BWG only at a supplementation level of 9ppm when they fed broiler chickens a basal diet containing 0.18mg Se/kg and supplemented with 0, 3, 6 and 9ppm. Similarly, in an experiment conducted on 1-day-old chicks fed a basal diet supplemented with Se (sodium selenite) at 0,2,5,10,15,20 and 30ppm for 6 weeks, TodorovicM, et al. [17] recorded a significant decrease in BWG only when the supplementation level was greater or equal to 5ppm.
In the above-mentioned studies, the supplementation level exceeds 1ppm. In some studies, using supplementation level less than 1ppm, authors did not also observe a significant effect on performance traits when broilers were fed diets supplemented with inorganic or organic Se at levels ranging from 0 to 0.6ppm [21-28]. On the other hand, Cantor AH, et al. [29] recorded higher live weight and increased feed intake after dietary Se (inorganic or organic) supplementation (0.04 to 0.12ppm). Dlouhá G, et al. [30] who studied a supplementation level of 0.3ppm of inorganic and organic Se in broiler chicken, stated that dietary supplementation with organic Se increased significantly body weight. HeindlJ, et al. [7]also confirmed that Se addition influenced body weight in 21- and 35-day-old broiler chickens. Significantly higher body weight at 35 days of age was determined in chickens fed diets supplemented with 0.15ppm and 0.3ppm of organic Se (Sel-Plex® SP).
As mentioned above, although there was no significant difference between dietary treatments in feed intake, this parameter was lowest in birds fed the D5 diet. This reduction appeared to be related to its high Se supplementation level (6ppm), which could have altered the taste of feed and make it unappetizing to animals. Indeed, Rosenfeld I, et al. [31] and Reily C[32] reported that at relatively high concentrations, Se compounds may affect the organoleptic characteristics of feed. In addition, the distribution of D5 was associated with the lowest BWG. This finding suggests a possible development of toxicity. Cantor AH, et al.[33] and Ibrahim MT, et al. [34] reported that the administration of Se enriched diets to broilers during the growth phase compromises their appetite and growth performance.
When correlation values between feed intake, BWG and FCR were calculated over the total duration of experiment (d12-40) and the Se content in the six diets tested, no significant correlation was observed between performance parameters and Se content of feed.
Se apparent balance
The determination of the apparent balance of Se was aimed at studying the influence of the incorporation of increasing levels of dietary organic Se on the retention of Se in whole carcass and its excretion in the environment. Thus, we calculated the amounts of Se ingested, retained and excreted expressed in mg/chicken. The results obtained are shown in Table 4a.
Table 4: Effect of Se dietary supplementation on ingested, retained, and excreted Se of broiler chickens during overall
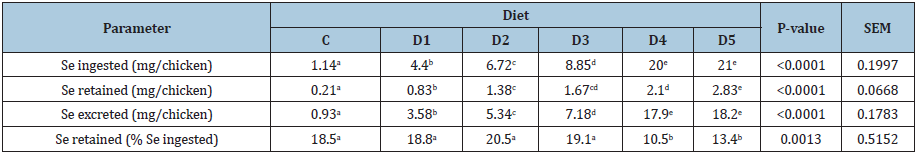
The Se ingested was significantly correlated with the Se content of the distributed feed (R2=98.13%, P <0.001). The amounts of Se ingested by birds fed D4 and D5 diets were statistically identical, but significantly higher than those calculated for birds receiving C, D1,D2, and D3 diets, which were also significantly different from each other. Regarding diets D4 and D5, although the difference between the two supplementation levels was equal to 2ppm, it was not surprising to find that their respective amounts of Se ingested were similar. Indeed, chickens fed D4 numerically consumed more feed than those fed D5.
Se retained corresponds to the difference between the amounts of Se contained in the whole carcasses of chicken on days d1 and d42. The amount of Se in the whole carcass corresponds to the product of the weight of the chickens by their contents in Se. The amount of Se contained in the whole carcass of 1-day-old chick averaged 10μg (data not shown). This value has been found to range between 11 and 13μg[35]. Regarding the Se content of the whole carcass of 42-day-old birds (Table 4b), the statistical analysis resulted in a positive linear relationship (R2=86.94%, P<0.001) between Se dietary supplementation level and Se content of the whole carcass. Whole carcass Se content increased significantly (P<0,0001) from 0.11mg/kg LBW (C) to 1.44mg/kg LBW (D5), i.e. an increase of 1.33mg. However, the Newman and Keuls test revealed that averages obtained with D2, D3 and D4 are statistically equivalent. This finding is consistent with the observation of Scott ML, Thompson JN [36], Cantor AH, et al. [29]and Echevarria MG, et al.[15,16]who argued that the inclusion of increasing amounts of Se in the chicken diet is associated with a significant increase in Se concentration in the tissues. Ševčíková S, et al. [23] and Ibrahim MT, et al. [34] respectively reported that breast, thigh, liver and plasma Se concentrations significantly increased with increasing Se levels in the diets. Nevertheless, our results indicate that at some point, the Se content in the carcass reached a saturation level. This finding suggests that stabilization of retention is one of the mechanisms involved in preventing excessive accumulation of Se in the body.
Table 4b: Effect of Se dietary supplementation on whole carcass Se content on d42 expressed in mg per kg of LBW and mg per chicken.
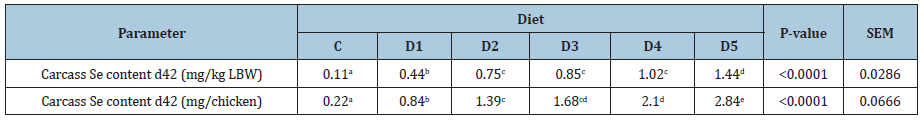
Se retention increased linearly and significantly (R2=97.13%, P<0,0001) with increasing Se dietary supplementation (Table 4a). Thus, the minimum Se retention was observed for the C diet (0.21mg/chicken) and the maximum one was observed for the D5 diet (2.83mg/chicken), which was nearly 14 times higher. Little information about the effect of Se dietary supplementation on Se content in whole carcass was published. Miller D, et al. [35], who used an incorporation level of 0.1 and 0.5mg Se/kg, observed a significant increase in Se retention in the carcass from 28 to 58.8μg/ chicken. Se retention values obtained by these authors are largely lower than that obtained for our control birds (220μg/chicken) who received 0.15mg Se/kg. i.e 3.6 times less, although authors also used an organic source of Se (selenomethionine). This difference could be explained by the fact that birds on which Se retention was determined were younger (21 days of age) and therefore probably lighter than ours (42-day-old birds).
In our study, the increase in Se concentration in whole carcass due to dietary Se supplementation is in agreement with findings presented in previous studies even that those researches focused on the assessment of Se retention in some tissues and not in the whole carcass. Accordingly, Scott ML, Thompson JN [36] and Echevarria MG, et al. [15,16] reported that Se concentration in the liver, kidneys, muscle, and plasma significantly increased linearly as Se content was increased in the diet. More recently, Ševčíková S, et al. [23], Dlouhá G, et al. [30], and SkřivanM, et al. [37] found that organic sources of selenium increased its content in breast meat. HeindlJ, et al. [37] confirmed that the Se content in breast muscle (0.6 and 0.85 mg/kg dry matter) was significantly increased by adding 0.15 and 0.30mg Sel-Plex® per kg of broilers diet in comparison with the Se-unsupplemented control (0.31mg/kg dry matter).,/
As cited above, the amount of Se ingested by chickens receiving D4 and D5 diets were statistically identical. We demonstrated that the body retention of Se was significantly higher in birds fed R5 diet. Therefore, there are potentially other factors besides the amount of ingested Se that influence the deposition of Se in the body.As indicated in Table 4a, we also expressed the amount of Se retained in percentage of that ingested (Se retained/Se ingested). This mode of expression makes it possible to evaluate the effectiveness of increasing the dietary supplementation level of Se. The highest ratios were recorded for C,D1,D3 and D2 and were equal to 18.5,18.8,19.1 and 20.6%, respectively. The lowest ones were obtained with D4 and D5:10.5 and 13.4% respectively. The Newman and Keuls test (Table 4a) reveals two mean groups differing significantly. The C,D1,D2 and D3 cluster on the one hand and D4 and D5 cluster on the other hand. Indeed, Se retention efficiency seems to be capped for a dietary supplementation level ranging between 0 and 3ppm, and to decrease beyond the threshold of 3ppm.
Overall, the selenium retention assessed in this trial was relatively low since the maximum retention was 20.6%. This result was unexpected since, in theory, selenomethionine is well retained by chicken [20,21,38-42]. Efficiency of absorption was not determined because this process is not subject to any regulatory mechanism [43,44] and the urinary route is the main route of excretion of Se [44,45]. This would indicate that totality of the ingested Se is necessarily supported by the metabolic process. The digestibility of the Se supplement (i.e., the yeast: Sel-Plex, Alltech. Inc) used in the current study could be the cause. Indeed, it has been reported that the amount of selenomethionine absorbed by the body depends on the digestibility of its dietary source [41]. Consequently, the assessment of the digestibility of Sel-Plex would be worth investigating.
It is also possible that, in our experiment, the dietary intake of methionine would have been very high, which could reduce the retention of selenomethionine. Indeed, data in the literature account for the competition between selenomethionine and methionine [38,41,46,47]. Thus, a high dietary intake of methionine inhibits the incorporation of selenomethionine, and thus interferes with its deposition in the body. The determination of methionine content in the yeast and the distributed feed would confirm our hypothesis. Our results also suggest that beyond a certain incorporation level of Se in the diet, poultry can no longer incorporate all of the ingested Se by accumulating it in the body. This would mean that there is a regulatory mechanism of Se retention in relation to the dietary intake of this trace element, and that Se in excess compared to the retention capacity is therefore excreted. This constitutes an economic loss on the one hand and a potential source of environmental pollution on the other.
Moreover, our observations demonstrated that Se dietary supplementation level of 0;1;2 and 3ppm are similar in terms of Se retention efficiency, thus Se dietary supplementation appears economically unfounded, especially when no significant improvement in growth performance was recorded for these supplementation levels. However, if the significant difference observed for the amount of Se retained in the whole carcass recorded for 2 and 3ppm levels is also reflected in the meat produced, the inclusion of these doses could be very interesting in the case of producing a high-Se meat. The determination of Se contained in the meat is therefore very useful.
Se excreted was determined by subtracting the amount of Se retained from that ingested. The statistical analyses demonstrated a significant linear effect (R2=86.96%, P<0.001) of Se dietary supplementation level on Se excretion. Indeed, the latter increased with the increase of Se incorporation level in the diet. In addition, it is positively correlated with the amount of Se ingested (R2=99.73%). This substantiates previous findings in the literature reporting increased excretion in case of increased dietary intake of Se. Indeed, Se homeostasis is maintained by the excretion of Se in excess [41,44,48].
The amount of Se excreted calculated in the present study did not account for any endogenous loss of Se. In poultry, this aspect, according to our knowledge, was not addressed in the literature. Nevertheless, in ruminants, LanglandsJP, et al. [49] reported that Se loss could occur via biliary secretion and it can reach up to 28% of the ingested fraction. A large part of the biliary Se is reabsorbed by the body, while the remainder is evacuated in feces. These authors also stated that endogenous faecal Se is positively correlated with ingested Se. Based on this information, excretion of Se calculated in this work may overestimate the true excretion of exogenous (dietary) Se.
Moreover, values shown in Table 4a do not include excretion in exhaled air: under normal circumstances, the absorbed Se is metabolized and then excreted in the urine as a trimethyl selenium ion. High concentrations can saturate this pathway and volatile Se compounds are formed and excreted via the lungs [44,45]. In addition, EdensFW [21,40], argues that Se is involved in the process of feathering. Thus, Se can also be eliminated during the natural losses of feathers.
Most modern poultry farms, which focus on mass production, produce huge amounts of droppings. Excessive accumulation of Se in droppings, usually used as fertilizer, can pose serious environmental problems; the most acute would be contamination of soil and groundwater. Thus, it seemed essential to evaluate the rejection of Se to get an opinion on the impact of Se dietary enrichment of broilers on the environment.
Meat lipids peroxidation
The evaluation of the peroxidation level of lipids in pectoral and thigh muscles was performed by the TBA index analysis. The measured TBA values, as a function of the Se dietary supplementation level, are reported in Table 5.
Table 5: Effect of Se dietary supplementation on pectoralis and thigh TBA index expressed in mg MDA/Kg.
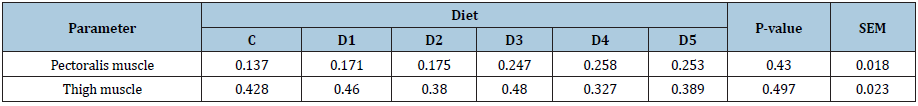
TBA index values measured in the muscle of the thigh are greater than those of the pectoralis muscle. Thus, the thigh muscles are more sensitive to lipid oxidation than the pectoral muscle. This observation is explained by the fact that in broilers, as for all poultry, the total lipid content of meat is higher in the muscles of the thigh (oxidative muscles) than in the breast (glycolytic muscles) [50].
The statistical analysis revealed no significant difference between the 6 diets for the TBA index, and whether it was in the pectoralis muscle or the thigh (P> 0.05). Furthermore, since in the present study, TBA values measured for the control birds were relatively low compared to those found in literature (ranging from 0,20 for the breast muscle to 0.5mg/kg for the thigh muscle), it is therefore possible that the meat produced was not rich enough in intramuscular fat to cause a significant phenomenon of peroxidation. Generally, at the end of the “finishing” phase, most of the increase in chicken’s weight is due to lipid deposition. Reflecting the fattening status of chickens, the weight of birds on which the muscle tissue was sampled can be used to detect a possible difference between the 6 diets concerning the lipid peroxidation level. Thus, the statistical analysis was rerun using LBW criterion as covariance with the same conclusion. Indeed, no significant difference was observed for the six diets, whether at the level of the pectoralis muscle (P = 0.430) or the thigh (P = 0.497).
The antioxidant effect of Se dietary supplementation on the stability of broiler chicken meat has been documented by a number of authors [30,37,51-53].The inclusion of 0.3mg/kg Se from Seenriched alga Chlorella in broilers diet enhanced the oxidative stability of meat expressed as reduced malondialdehyde values in breast meat after a 0-,3-, and 5-day refrigeration at 3-5°C[30]. SkřivanM [37] confirmed these findings in a similar study using 0.3mg/kg Selenomethionine. In this respect, Pappas AC, et al. [54] also showed that inclusion of Se-yeast in broilers diet at level of 0.15, 0.3 and 3mg/kg was associated with a significant linear decrease in TBRAS content of meat as an indicator of a decrease in lipid oxidation at slaughter.
Similarly, Mikulski D, et al. [55] found that dietary supplementation with 0.3mg/kg of Se yeast resulted in a significant (P<0.05) decrease in thiobarbituric acid reactive substance in raw (24h after slaughter) and stored meat (stored at-20°C for 70 days, thawed and stored for a further 3 days at 4°C) of turkey. TBA index in the pectoralis muscle was significantly and moderately correlated with the increase of Se dietary supplementation level (R2=37%, P=0.007). This positive and moderate correlation remains unexplained, unless the increase of the dietary Se would have had a pro-oxidant action. Indeed, Lee KH & Jeong D [56] reported that high intake of Se even in organic form could have a pro-oxidative action. This could be due to the release of Se during the post-mortem evolution of the muscle into meat. During this phase, muscle proteins, including those containing selenomethionine, undergo enzymatic hydrolysis under the action of calpine and cathepsin. After the catabolism of selenomethionine, Se can be released. Thus, it is possible that it had a pro-oxidative action on muscle lipids. However, if this were the case, we should have observed this effect and more markedly with the thigh muscles which are more sensitive to oxidation than the pectoralis muscles. However, the correlation between TBA index values measured on the thigh with the dietary Se content in the diet was very weak and no significant (R2=6.5%, p=0.308).
A second hypothesis related to the post-mortem mechanisms that can take place in the muscle and modify its degree of lipids peroxidation as lipolysis. This mechanism is catalysed by specific enzymes such as lipases and phospholipases [50]. According to the same review, an excessive increase in the activity of these enzymes, particularly that of phospholipases, results in an increase in the fraction of long-chain PUFAs in muscle lipids. This induces an increase in the rate of peroxidation of these lipids. The cause of overproduction of phospholipases can be attributed to certain nutritional interactions, which are not yet well defined [50].
The assessment of the Glutathione peroxidase (GSH-Px) activity at the muscle level could have provide an explanation to results obtained during our experimentation. Indeed, the GSH-Px is an enzyme transforming the excessive peroxide and hydrogen peroxides of fatty acids resulting from oxidative elimination of lipids to harmless water and oxygen [44,57]. Its activation requires small amounts of Se (selenocysteine) which represents its integral part [58-60]. It is documented in the literature that Se dietary supplementation increased GSH-Px activity in plasma and muscle of broilers [7,27,29,30,61].
Conclusion
Our results suggested that in Cobb broiler chicken, the inclusion of organic Se at a level of 3mg/kg in a conventional growth-finisher diet containing 0.15mg Se/kg of feed improved slightly their BWG and FCR (+2.5% and -2.8%, respectively). Se carcass retention increased in a dose-dependent manner, but retention efficiency was very low. Increasing Se dietary level resulted in no effect on oxidative status in breast and thigh meat after 7-day storage period at 4°C.
References
- Kieliszek M, Błazejak S (2016) Current knowledge on the importance of selenium in food for living organisms: A review. Molecules 21: 609.
- Ministry of Public Health (2018) National survey on hospital morbidity and mortality, Tunisia.
- Surai PF (2006) Selenium in nutrition and health. Nottingham University Press, Nottingham, UK.
- Wolffram S, Anliker E, Scharrer E (1986) Uptake of selenate and selenite by isolated intestinal brush border membrane vesicles from pig, sheep, and rat. Biological Trace Element Research 10: 293-306.
- Briens M, Mercier Y, Rouffineau F, Vacchina V, Geraert PA (2013) Comparative study of a new organic selenium source v. seleno-yeast and mineral selenium sources on muscle selenium enrichment and selenium digestibility in broiler chickens. Br J Nutr 110: 617-
- Ellis DR, Salt DE (2003) Plants, selenium and human health. Current Opinion in Plant Biology 6: 273-279.
- Heindl J, Ledvinka Z, Englmaierová M, Zita L, Tumová E (2010) The effect of dietary selenium sources and levels on performance, selenium content in muscle and glutathione peroxidase activity in broiler chickens. Czech Journal of Animal Science 55: 572-578.
- Gao X, Xing H, Li S, Li J, Ying T, et al. (2012) Selenium regulates gene expression of selenoprotein W in chicken gastrointestinal tract. Biological Trace Element Research 145: 181-188.
- National Resarch Council (1994) Nutrient Requirements of Poultry. (9th rev ed). National Academy Press, Wasshington, USA.
- Bohrer D, Becker E, Nascimento CP, Dessuy M, de Carvalho LM (2007) Comparison of graphite furnace and hydride generation atomic absorption spectrometry for the determination of selenium status in chicken meat. Food Chemistry 104: 868-875.
- Gomes de AH, da Silva EN, do Nascimento MRL, Fukuma HT (2003) Evaluation of the 2-thiobarbituric acid method for the measurement of lipid oxidation in mechanically deboned gamma irradiated chicken meat. Food chemistry 80: 433-437.
- SAS Institute (1990) SAS Institute SAS/STAT User's Guide: Statistics. SAS Institute Inc, NC, USA.
- JORT (1996) The Official Journal of the Republic of Tunisia.
- Suchý P, Straková E, Herzig I (2014) Selenium in poultry nutrition: A review. Czech Journal of Animal Science 59: 495-503.
- Echevarria MG, Henry PR, Ammerman CB, Rao PV, Miles RD (1988a) Estimation of the relative bioavailability of inorganic selenium sources for poultry. 1. Effect of time and high dietary selenium on tissue selenium uptake. Poultry Science 67: 1295-1301.
- Echevarria MG, Henry PR, Ammerman CB, Rao PV, Miles RD (1988) Estimation of the relative bioavailability of inorganic selenium sources for poultry. 2. Tissue uptake of selenium from high dietary selenium concentrations. Poultry Science 67: 1585-1592.
- Todorovic M, Mihailovic M, Hristov S (1999) Effects of excessive levels of sodium selenite on daly weight gain, mortality and plasma selenium concentration in chickens. Acta Veterinaria 49: 313-319.
- Deniz G, Genzen SS, Turkmen II (2005) Effects of two supplemental dietary selenium sources (mineral and organic) on broiler performance and drip-loss. Revue de Medecine Veterinaire 156: 423-426.
- Salyi G, Banhidi G, Szabo E, Gonye S, Ratz F (1993) Acute selenium poisoning in broilers. Magy Allatorvosok 48: 22-26.
- Surai PF (2002) Natural antioxidants in avian nutrition and reproduction. Nottingham Univeersity Press, Nottingham, UK.
- Edens FW (1996) Organic selenium: from feathering to muscle integrity to drip loss. Five years onward: No more selenite. In: Lyons TP, Jacques KA (Eds.) Biotechnology in feed Industry. Proceedings of the 12th Annual Symposium Nottingham University Press, Nottingham, UK, pp. 349-370.
- Downs K, Hess J, Bilgili S (2000) Selenium source effect on broiler carcass characteristics, meat quality and drip loss. Journal of Applied Animal Research 18: 61-72.
- Ševčíková S, Skřivan M, Dlouhá G, Koucký M (2006) The effect of selenium source on the performance and meat quality of broiler chickens. Czech Journal of Animal Science 51: 449-457.
- Peric L, Milosevic N, Zikic D, Kanacki Z, Dzinic N, et al. (2009) Effect of selenium sources on performance and meat characteristics of broiler chickens. Journal of Applied Poultry Research 18: 403-409.
- De Medeiros LG, Oba A, Shimokomaki M, Pinheiro JW, Da Silva CA, et al. (2012) Performance, broiler carcass and meat quality characteristics, supplemented with organic selenium. Semina-Ciencias Agrarias 33: 3361-3370.
- Chen G, Wu J, Li C (2013) The effect of different selenium levels on production performance and biochemical parameters of broilers. Italian Journal of Animal Science 12: 486-491.
- Rama Rao SV, Prakash B, Raju MVLN, Panda AK, Poonam S, et al. (2013) Effect of supplementing organic selenium on performance, carcass traits, oxidative parameters and immune responses in commercial broiler chickens. Asian Australasian Journal of Animal Sciences 26: 247-252.
- Oliveira T, Rivera D, Mesquita F, Braga H, Ramos E, et al. (2014) Effect of different sources and levels of selenium on performance, meat quality, and tissue characteristics of broilers. Journal of Applied Poultry Research 23: 15-22.
- Cantor AH, Moorhead PD, Musser MA (1982) Comparative effects of sodium selenite and selenomethionine upon muscular dystrophy, selenium-dependent glutathione peroxidase and tissue selenium concentrations of turkey poults. Poultry Science 61: 478-484.
- Dlouhá G, Ševčíková S, Dokoupilová A, Zita, L, Heindl J, et al. (2008) Effect of dietary selenium sources on growth performance, breast muscle selenium, glutathione peroxidase and oxidative stability in broilers. Czech Journal of Animal Science 53: 265-269.
- Rosenfeld I, Beath OA (1964) Selenium: geobotany, biochemistry, toxicity, and nutrition. Academic Press, New York.
- Reilly C (1998) Selenium: A new entrant into the functional food arena. Trends in Food Science and Technology 9: 114-118.
- Cantor AH, Nash DM, Johnson TH (1984) Toxicity of selenium in drinking water of poultry. Nutrition Reports International 29: 683-688.
- Ibrahim MT, Eljack BH, Fadlalla IMT (2011) Selenium supplementation to broiler diets. Animal Science Journal 2: 12-17.
- Miller D, Soares JH, Bauersfeld P, Cuppett SL (1972) Comparative selenium retention by chiks fed sodium selenite, selenomethionine, fish meal and fish solubles. Poultry Science 51: 1669- 1673.
- Scott ML, Thompson JN (1971) Selenium content of feedstuffs and effects of dietary selenium levels upon tissue selenium in chiks and poults. Poultry Science 50: 1742-1748.
- Skřivan M, Dlouhá G, Mašata O, Ševčíková S (2008) Effect of dietary selenium on lipid oxidation, selenium and vitamin E content in the meat of broiler chickens. Czech Journal of Animal Science 53: 306-311.
- Mahan DC (1994) Organic selenium sources for swine-How do they compare with inorganic selenium sources? In: Lyons TP, Jacques KA (Eds.). Biotechnology in feed Industry. Proceedings of the 13th Annual Symposium. Nottingham University Press, Nottingham, UK, pp. 323-333.
- Mahan DC (1995) Selenium metabolism in animals: What role does selenium yeast have? In: Lyons TP, Jacques KA (Eds.). Biotechnology in feed Industry. Proceedings of the 11th Annual Symposium. Nottingham University Press, UK, pp. 257-267.
- Edens FW (2001) Involvement of Sel-Plex in physiological stability and performance of broiler chickens. In: Lyons TP, Jacques KA (Eds.). Biotechnology in feed Industry. Proceedings of the 17th Annual Symposium. Nottingham University Press, Nottingham, UK, pp. 349-370.
- Jacques KA (2001) Selenium metabolism in animals: In: The relationship between dietary selenium form and physiological function. Science and Technology in the Feed Industry. In: Jacques KA and Lyons TP (Eds.). Nottingham University Press, United Kingdom, pp. 319-348.
- Wang YB, Xu BH (2008) Effect of different selenium source (sodium selenite and selenium yeast) on broiler chickens. Animal Feed Science and Technology 144: 306-314.
- Combs Jr GF, Combs SB (1986) The Role of Selenium in Nutrition. Academic Press, New York.
- Burk RF (2002) Selenium, an antioxidant nutrient. Nutrition in Clinical Care 5: 75-79.
- Nève J, Therond P (1991) Le sélé In: Les oligoéléments en médecine et biologie, Lavoisier - Tec Doc (Eds), Paris, France, pp. 425-454.
- Wolffram S (1999) Absorption and metabolism of selenium: Differences between inorganic and organic sources. In: Lyons TP, Jacques KA (Eds.). Biotechnology in feed Industry. Proceedings of the 1st Annual Symposium. Nottingham University Press, Nottingham, UK, pp. 547-566.
- Stahl W, Van Den BH, Arthur J, Bast A, Dainty J, et al. (2002) Bioavailability and metabolism. Molecular Aspects of Medicine 23: 39-100.
- Alarcon NM, Martinez LMC (2000) Essentiality of selenium in the human body: Relationship with different diseases. The Science of the Total Environment 249: 347-371.
- Langlands JP, Bowles JE, Donald GE, Smith AJ (1986) Selenium excretion in sheep. Australian Journal of Agricultural Research 37: 201-209.
- Gandemer G, Meynier A, Genot C (1997) Phospholipids-Lipolysis, oxidation and flavour of meat. In: Berger KG, Barnes PJ, Associates (Eds.). Animal fats - BSE and After. Bridgwater, UK, 7: pp. 119-136.
- Wang YX, Zhan XA, Yuan D, Zhang XW, Wu RJ (2011) Effects of selenomethionine and sodium selenite supplementation on meat quality, selenium distribution and antioxidant status in broilers. Czech Journal of Animal Science 56: 305-313.
- Liao X, Lu L, Li S, Liu S, Zhang L, et al. (2012) Effects of selenium source and level on growth performance, tissue selenium concentrations, antioxidation, and immune functions of heat-stressed broilers. Biological Trace Element Research 150: 158-165.
- Yang YR, Meng FC, Wang P, Jiang YB, Yin QQ, et al. (2012) Effect of organic and inorganic selenium supplementation on growth performance, meat quality and antioxidant property of broilers. African Journal of Biotechnology 11: 3031-3036.
- Pappas AC, Zoidis E, Papadomichelakis G, Fegeros K (2012) Supranutritional selenium level affects fatty acid composition and oxidative stability of chicken breast muscle tissue. Journal of Animal Physiology and Animal Nutrition 96: 385-394.
- Mikulski D, Jankowski J, Zdunczyk Z, Wróblewska M, Sartowska K, et al. (2009) The effect of selenium source on performance, carcass traits, oxidative status of the organism, and meat quality of turkeys. Journal of Animal Feed Science 18: 518-530.
- Lee KH, Jeong D (2012) Bimodal actions of selenium essential for antioxidant and toxic pro-oxidant activities: the selenium paradox (review). Molecular Medicine Reports 5: 299-304.
- De Almeida JN, Dos Santos GR, Beteto FM, De Medeiros LG, Oba A, et al. (2012) Dietary supplementation of chelated selenium and broiler chicken meat quality. Semina Ciencias Agrarias 33: 3117-3122.
- Mills GC (1957) Hemoglobin metabolism I. Glutathione peroxidase, an erytrocyte enzyme which protect hemoglobin from oxidative damage. The Journal of Biological Chemistry 229: 189-197.
- Flohe L, Günzler WA, Schock HH (1973) Glutathione peroxidase: A selenoenzyme. FEBS Letters 32: 132-134.
- Rotruck JT, Pope AL, Ganther HE, Swanson AB, Hafeman DG, et al. (1973) Selenium: biochemical role as a component of glutathione peroxidase. Science 179: 588-590.
- Cai S, Wu C, Gong L, Song T, Wu H, (2012) Effects of nano-selenium on performance, meat quality, immune function, oxidation resistance, and tissue selenium content in broilers. Poultry Science 91: 2532-2539.
© 2020 R Chalghoumi. This is an open access article distributed under the terms of the Creative Commons Attribution License , which permits unrestricted use, distribution, and build upon your work non-commercially.
 a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in
a Creative Commons Attribution 4.0 International License. Based on a work at www.crimsonpublishers.com.
Best viewed in 







.jpg)






























 Editorial Board Registrations
Editorial Board Registrations Submit your Article
Submit your Article Refer a Friend
Refer a Friend Advertise With Us
Advertise With Us
.jpg)






.jpg)














.bmp)
.jpg)
.png)
.jpg)










.jpg)






.png)

.png)



.png)






